What is the pH and pOH of a solution that was made by adding 245 mL of water to 500 mL of 4 * 10 ^ - 4 M HF solution?
PH?round to 1 decimal place
pOH 1 decimal place
Answers
The pH is 3.9
The pOH is 10.1
How to solve for pH and pOHFind the total volume first
Total volume = 500 mL + 245 mL
= 745 mL
Find the Moles of HF
= (4 * 10^-4 M) * (0.500 L)
= 2 * 10^-4 moles
Find Molarity of diluted solution
= (2 * 10^-4 moles) / (0.745 L)
= 2.68 * 10^-4 M
Ka of HF is 6.8 * 10^-4
the expression = 6.8 * 10^-4 = x^2 / (2.68 * 10^-4)
solve for x
x^2 = 1.82 * 10^-7
x = 1.35 * 10^-4
Then the pH
= -log10(1.35 * 10^-4)
pH ≈ 3.9
The pOH
pH + pOH = 14
pOH = 14 - 3.9
= 10.1
Read more on pH and pOH here:https://brainly.com/question/1420583
#SPJ1
Related Questions
ASAP!!!!!! PLSS
This question has two parts. First, answer Part A. Then, answer Part B.
Part A
Fill in the blank question.
When as 30.98-g sample of phosphorus reacts with oxygen, a 71.00-g sample of phosphorus oxide is formed. What is the percent composition of the compound?
How many grams of phosphorus are in a 100.0g sample of the phosphorus oxide?
What percent of the phosphorus oxide is phosphorus?
What percent of the phosphorus oxide is oxygen?
Part B
Fill in the blank question.
What is the empirical formula for this phosphorus oxide compound?
Step 2:
How many moles of phosphorus?
How many moles of oxygen?
Step 3: Divide by the smallest # moles, make whole numbers.
What is the subscript for the phosphorus?
What is the subscript for oxygen?
Answers
The chemical has a percent composition of 56.35% oxygen and 43.65% phosphorus.
What is the empirical formula for a substance that contains 11.1% hydrogen and 88.9% oxygen?A substance has an oxygen content of 88.79% and a hydrogen content of 11.19%. Calculate this compound's empirical formula. Nonetheless, it's customary to employ the atom ratio with the smallest whole number. H 2 O is the compound's empirical formula as a result.
Determine the compound's overall mass:P mass of 30.98 g and O mass of 40.00 g add up to 70.98 g.
Phosphorus: \((30.98 g / 70.98 g) x 100% = 43.65%\)
\((40 g/70.98 g) x 100% = 56.35% for oxygen.\)
To know more about phosphorus visit:-
https://brainly.com/question/4622631
#SPJ1
help me because I'm the coolest person in here right behind myself

Answers
Answer:
Explanation:
5. Deuterium, also known as heavy hydrogen, is a Hydrogen isotope with mass number 2.
6. Isotope is an atom of the same element with the same number of protons but different number of neutrons.
The data found below measure the amounts of greenhouse gas emissions from three types of vehicles. The measurements are in tons per year, expressed as CO2 equivalents. Use a 0.025 significance level to test the claim that the different types of vehicle have the same mean amount of greenhouse gas emissions. Based on the results, does the type of vehicle appear to affect the amount of greenhouse gas emissions? Click the icon to view the data. What are the hypotheses for this test? A. H 0
:μ 1
=μ 2
=μ 3
H 1
: At least one of the means is different from the others. B. H 0
: At least one of the means is different from the others. H 1
:μ 1
=μ 2
=μ 3
C. H 0
:μ 1
=μ 2
=μ 3
H 1
:μ 1
=μ 2
=μ 3
D. H 0
:μ 1
=μ 2
=μ 3
H 1
:μ 1
=μ 2
=μ 3
Determine the test statistic. F (Round to two decimal places as needed.)
Answers
Answer: A. H 0 μ1 = μ2 = μ3
Ha μ1 ≠ 2μ ≠ μ3
2. Test Statistics is 95%
3. Critical F-Value is 3.76.
4. P-Value is 2.32.
5. Conclusion Reject the null hypothesis.
6. Type of vehicle does effect the amount of green house gas emissions.
The correct order of the steps of a hypothesis test is given below.
1. Determine the null and alternative hypothesis.
2. Select a sample and compute the critical value F-test for the sample mean.
3. Determine the probability at which you will conclude that the sample outcome is very unlikely.
4. Make a decision about the unknown population.
All steps are performed in the given sequence to test a hypothesis.
The null hypothesis is rejected or accepted on the basis of level of significance. When the p-value is greater than level of significance we fail to reject the null hypothesis and null hypothesis is then accepted. It is not necessary that all null hypothesis will be rejected at 95% level of significance. To determine the criteria for accepting or rejecting a null hypothesis we should also consider p-value.
For more details on hypothesis test follow the link:
brainly.com/question/10758924
Explanation:
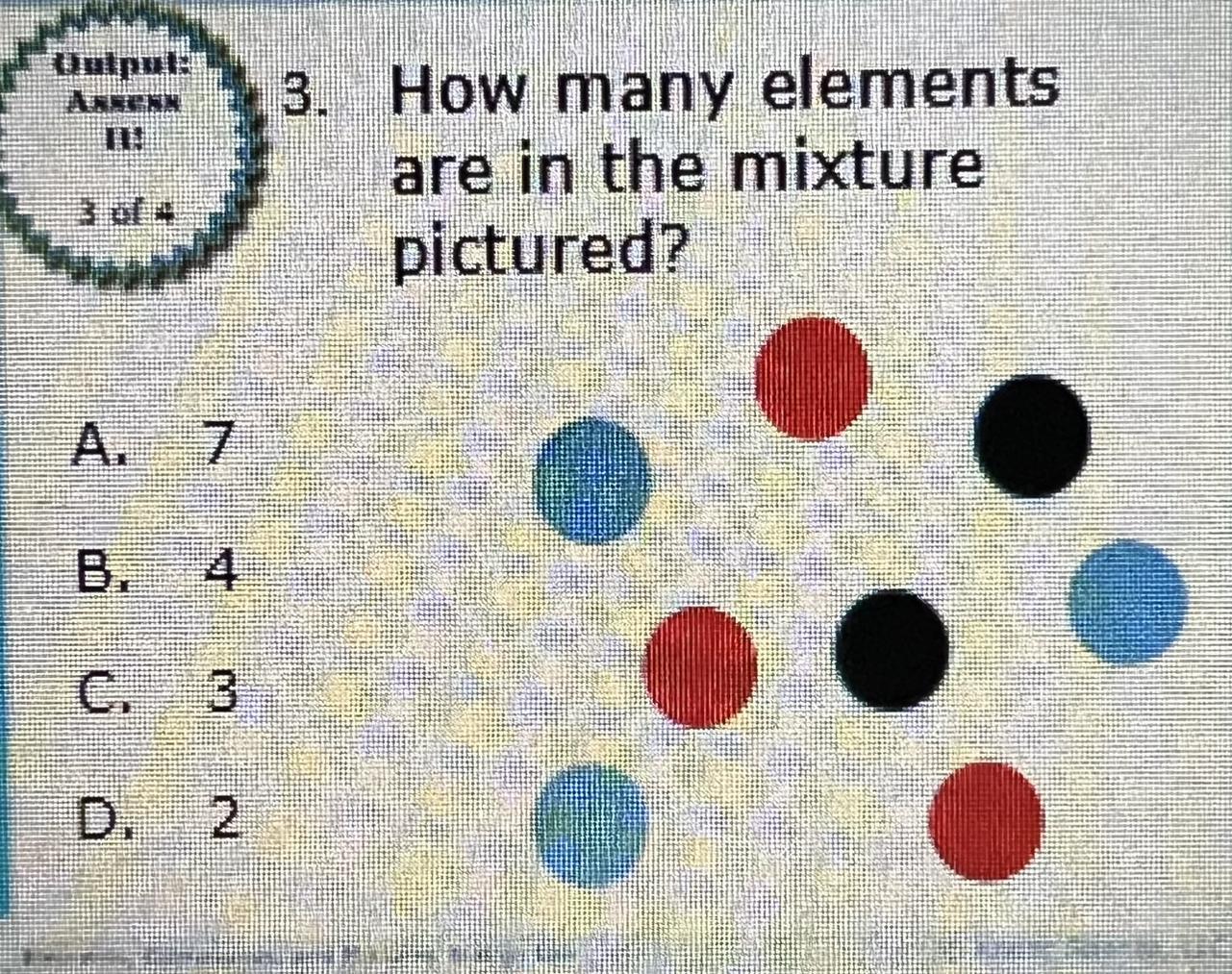
How many elements
are in the mixture
pictured?
A. 7
B. 4
C. 3
D. 2
Answers
There are 3 elements, (option C) is the right option.
The element can be defined as one of the simplest chemical substances that cannot be decomposed in a chemical reaction and are made up of atoms all having the same number of protons.
As stated above, all elements are made up of backbone structures called atoms. The atoms of an element are the same.
According to this question, an image is given showing atoms of different colors. Each atom has the same color and belongs to the same element.
Since there are there colors, this means that there are three elements in the picture.
read more about elements
brainly.com/question/25916838
HD:pSun =rhoMan =pTue =pWed =pThu =pFri =pSat =71 Ha : Not all proportions are equal. HD: Not all proportions are equal. Ha:pSun =pMon =pTue =rhoWed =pThu =rhoFri =rhoSat =71 HD: Not all proportions are equal. Ha:pSun =pMon =pTue =pWed =pThu =rhoFri =pSat =71 HD:pSun =pMon =pTue =pWod =pThu =pFri =pSat =71 Ha : All proportions are equal. Find the value of the test statistic. (Round your answer to three d: Find the p-value. (Round your answer to four decimal places.) p-value = State your conclusion. Do not reject H0−. We conclude that the proportion of traffic Reject HD. We conclude that the proportion of traffic acciden Reject HD. We conclude that the proportion of traffic acciden Do not reject H0−We conclude that the proportion of traffic Compute the percentaqe of traffic accidents occurring on each day What day has the highest percentage of traffic accidents? Sunday Monday Tuesday Wednesday Thursday Friday Saturday Based on 2017 sales, the six top-selling compact showed the following number of vehicles sold. Use a goodness of fit test to determine if the sample data indicate that the market shares for compact cars in the city are different than the market shares suggested by nationwide 2017 sales. Use a 0.05 level of significance. State the null and alternative hypothesis. Ha : The market shares for the compact cars in the city are different for at least one of the nationwide market shares listed. o: The market shares for the compact cars in the city do not differ from market shares nationwide. : The market shares for the compact cars in the city are different from at least one of the nationwide market shares listed. Ha : The market shares for the compact cars in the city are not different from any of the natione Ha : The market shares for the compact cars in the city do not differ from market shares nationwide. "the test statistic.(Round your answer to two decimal places.) d the rho-value. (Round your answer to four decimal places.) Reject H0. We cannot conclude that market shares for the compact cars in the city differ from the nationwide market shares. Do not reject H0. We conclude that market shares for the compact cars in the city differ from the nationwide market shares. Do not reject H0. We cannot conclude that market shares for the compact cars in the city differ from the nationwide market shares.
Answers
The p-value is greater than the significance level of 0.05, we do not reject the null hypothesis and conclude that all proportions are equal.
Firstly, let us conduct a Chi-square test of independence of categorical variables based on the information given above. We have three different cases of hypothesis testing that we have to solve one by one.
Case 1: HD:pSun =rhoMan =pTue =pWed =pThu =pFri =pSat =71
Ha : Not all proportions are equal.
Test Statistic
For this hypothesis, we need to compute the test statistic that is given as:
\($$\chi^2=\sum_{i=1}^{k}\frac{(O_i-E_i)^2}{E_i}$$\) where k is the number of groups/categories. Since we have 7 days of the week, \(k = 7. $O_i$ and $E_i$\) are the observed and expected frequencies respectively.
Here, we have equal proportions of 71 for each day of the week.
Therefore, the expected frequencies are also equal to 71.
\($$E_i = 71, i=1,2,3,4,5,6,7.$$\)
We also have to use the given information to compute the observed frequencies,
\($O_i$.$$O_1 = 90, O_2 = 99, O_3 = 122, O_4 = 123, O_5 = 130, O_6 = 160, O_7 = 126$$\)
Therefore, the test statistic can be computed as \($$\chi^2=\frac{(90-71)^2}{71} + \frac{(99-71)^2}{71} + \frac{(122-71)^2}{71} + \frac{(123-71)^2}{71} + \frac{(130-71)^2}{71} + \frac{(160-71)^2}{71} + \frac{(126-71)^2}{71}$$$$\chi^2=180.14\)
Now we have to find the p-value of this test. Since the number of degrees of freedom is k - 1 = 7 - 1 = 6, the p-value can be found using the chi-square distribution table with 6 degrees of freedom at the 0.05 significance level. The p-value is 0.000014. ConclusionSince the p-value is less than the significance level of 0.05, we reject the null hypothesis and conclude that not all proportions are equal.
The total number of accidents is \($$90+99+122+123+130+160+126=850$$\)
The percentage of accidents occurring on each day of the week can be found as follows:
\($$Sunday: $$\frac{90}{850}\times 100 = 10.59\%$$Monday: $$\frac{99}{850}\times 100 = 11.65\%$$Tuesday: $$\frac{122}{850}\times 100 = 14.35\%$$Wednesday: $$\frac{123}{850}\times 100 = 14.47\%$$Thursday: $$\frac{130}{850}\times 100 = 15.29\%$$Friday: $$\frac{160}{850}\times 100 = 18.82\%$$Saturday: $$\frac{126}{850}\times 100 = 14.82\%$$\)
From the above percentages, we can see that Friday has the highest percentage of traffic accidents.
Case 2:
HD: Not all proportions are equal.
Ha:pSun =pMon =pTue =rhoWed =pThu =rhoFri =rhoSat =71
Test Statistic
\($$E_1 = 78.57, E_2 = 86.57, E_3 = 106.86, E_4 = 107.43, E_5 = 113.57, E_6 = 139.43, E_7 = 109.14$$\)
We already know the observed frequencies,
\($$O_1 = 90, O_2 = 99, O_3 = 122, O_4 = 123, O_5 = 130, O_6 = 160, O_7 = 126.$$\)
The test statistic can be computed as:
\($$\chi^2=\frac{(90-78.57)^2}{78.57} + \frac{(99-86.57)^2}{86.57} + \frac{(122-106.86)^2}{106.86}+ \frac{(123-107.43)^2}{107.43} + \frac{(130-113.57)^2}{113.57} + \frac{(160-139.43)^2}{139.43} + \frac{(126-109.14)^2}{109.14} $$$$ \implies \chi^2=34.98$$\)
The p-value is 0.000001.
Conclusion- Since the p-value is less than the significance level of 0.05, we reject the null hypothesis and conclude that not all proportions are equal.
Case 3:
All proportions are equal.
Test Statistic
The expected frequency for each group is
\(E = \frac{850}{7} = 121.43\)
We already know the observed frequencies,
\($$O_1 = 90, O_2 = 99, O_3 = 122, O_4 = 123, O_5 = 130, O_6 = 160, O_7 = 126.$$\)
The test statistic is,
\($$\chi^2=\frac{(90-121.43)^2}{121.43} + \frac{(99-121.43)^2}{121.43} + \frac{(122-121.43)^2}{121.43} + \frac{(123-121.43)^2}{121.43} + \frac{(130-121.43)^2}{121.43} + \frac{(160-121.43)^2}{121.43} + \frac{(126-121.43)^2}{121.43}} \\\implies \chi^2=9.17$$\)
The p-value is 0.1664.
Since the p-value is greater than the significance level of 0.05, we do not reject the null hypothesis and conclude that all proportions are equal.
To know more about p-value visit:
https://brainly.com/question/30761573
#SPJ11
The ph of a 0.15 m aqueous solution of nabro (the sodium salt of hbro) is 10.7. what is the k a for hbro?
Answers
The Ph of a 0.15 m aqueous solution of Na Br O (the sodium salt of H Br O) is 10.7. what is the k a for H Br O is 6.0\(E^{-9}\).
What is an aqueous solution?An aqueous solution is a solution in which solvent is water. It is mostly shown in chemical equations by appending (a q) to relevant chemical formula. For example, solution of table salt, or sodium chloride (NaCl), in water would be represented as Na+(a q) + Cl−(a q). The word aqueous (which comes from aqua) means pertaining to, related to, similar to, or dissolved in, the water. As water is an excellent solvent and is also naturally abundant, it is ubiquitous solvent in chemistry. Since water is frequently used as solvent in experiments, the word solution refers to an aqueous solution, unless the solvent is specified.
A non-aqueous solution is a solution in which solvent is a liquid, but is not water.
Reactions in aqueous solutions are usually the metathesis reactions. Metathesis reactions are another term for the double-displacement; that is, when a cation displaces to form an ionic bond with the other anion. The cation bonded with latter anion will dissociate and bond with the other anion.
A common metathesis reaction in the aqueous solutions is a precipitation reaction. This reaction occurs when two aqueous strong electrolyte solutions mix and produce insoluble solid, also known as a precipitate. The ability of a substance to dissolve in water is determined by whether substance can match or exceed the strong attractive forces that water molecules generate between themselves. If the substance lacks the ability to dissolve in water, molecules form a precipitate.
When writing the equations of precipitation reactions, it is essential to determine precipitate. To determine the precipitate, one must consult chart of solubility. Soluble compounds are aqueous, while the insoluble compounds are the precipitate. There may not always be precipitate. Complete ionic equations and net ionic equations are used to show the dissociated ions in metathesis reactions. When performing the calculations regarding the reacting of one or more aqueous solutions, in general one must know the concentration, or molarity, of the aqueous solutions.
To know more about molarity visit: https://brainly.com/question/8732513
#SPJ4
what is the empirical formula of stannous fluoride, the first fluoride compound added to toothpaste to protect teeth against decay? its mass percent composition is 24.25% ff , 75.75% snsn .
Answers
The empirical formula for stannous fluoride, a substance added to toothpaste to prevent tooth decay, is SnF2, if its mass percentage composition is 24. 25% F, 75.
Sn 75.75 119/75.75m= 1\s F 24.25 19/24.25 = 2
SnF2 is the resultant compound.
Thus, the information provided is accurate.
You must first determine the mass percentage of the elements in the compound for any empirical formula problem.
Change the% to grammes after that.
Next, split each mass by its associated molar mass.
Divide all numbers by the moles response that is the least.
The chemical compound known as stannous fluoride, sometimes known as tin(II) fluoride (from the Latin stannum, "tin"), has the formula SnF2. It is a colourless solid that is utilised as a toothpaste ingredient.
To learn more about fluoride please click on below link
https://brainly.com/question/2807538
#SPJ4
help pls!!
H2O(g) → H2O(l)
The chemical equation above describes a(n) __________.
A) irreversible state change
B) physical change
C) endothermic change
D) chemical change
Answers
Answer:
C, physical change
Explanation:
take a look at the equation. Next to each form of water do you see the little (g) and (l)? These represent the state of matter the water is in. So, the water is going from a gas to a liquid. The chemical compound is the same so its a physical change, not chemical, and it is reversible. You could change the liquid water back to the gas through evaporation. Endothermic has literally nothing to do with this equation so you can easily eliminate that answer choice.
what reaction (oxidation or reduction) occurs at the cathode of a voltaic cell?
Answers
In a voltaic cell, the reaction that occurs at the cathode is the reduction reaction.
A voltaic cell consists of two half-cells, each containing an electrode (cathode or anode) and an electrolyte. In the cell, electrons flow from the anode to the cathode through an external circuit, generating electricity. At the cathode, reduction takes place as the positively charged ions in the electrolyte gain electrons, leading to a decrease in their oxidation state.
A voltaic cell, often called a galvanic cell, is an electrochemical device that produces electricity through uninhibited redox processes, both oxidation and reduction. It is divided into two distinct half-cells. A half-cell is made up of an electrode (a metal strip, M) dissolved in a solution containing Mn+ ions. M can be any metal. A wire from one electrode to the other connects the two half-cells cathode and anode. The half-cells are also connected by a salt bridge.
To learn more about voltaic cell, visit:
https://brainly.com/question/26228780
#SPJ11
Titaium oxside is often added to food to color it white. If a jelly bean contains approximately 1.28x10-5 moles of TiO2, how many grams of TiO2 are in a jelly bean
Answers
Answer:
1.02 × 10⁻³ g
Explanation:
Step 1: Given data
Number of moles of titanium (IV) oxide in 1 jelly bean (n): 1.28 × 10⁻⁵ moles
Step 2: Calculate the mass (in grams) corresponding to 1.28 × 10⁻⁵ moles of TiO₂
To convert moles to mass, we need a conversion factor. In this case, it is the molar mass of TiO₂: 79.87 g/mol.
1.28 × 10⁻⁵ mol TiO₂ × 79.87 g TiO₂/1 mol TiO₂ = 1.02 × 10⁻³ g TiO₂
Which describes a molecule? (Select all that apply.)
It can be two or more different elements combined.
It can be two or more of the same elements combined together.
It can be one element by itself.
It can be the combination of different neutrons.
Answers
Answer:
All of them
Explanation: A molecule can be all of them
How many electrons will an element have if it has an atomic number of 20, an
atomic mass of 40 and a charge of +2? *
A.)40
B.)18
C.)22
D.)20
Answers
This is because the atomic number is the amount of protons. The number of protons equal the number of electrons in a neutral atom. But since it has a charge of +2, you subtract 2 from 20. +2 is a anion.
calculate the mvolume of a o.35 m naf solution which would be needed to completely react with 869 ml of a 0.38 m srcl2 solution
Answers
1890 mL of a 0.35 M NaF solution would be needed to completely react with 869 mL of a 0.38 M SrCl2 solution.
The balanced chemical equation for the reaction between sodium fluoride (NaF) and strontium chloride (SrCl2) is:
2NaF + SrCl2 → 2NaCl + SrF2
According to the equation, 2 moles of NaF are required to react with 1 mole of SrCl2.
First, we need to calculate the number of moles of SrCl2 present in 869 mL of a 0.38 M solution:
0.869 L x 0.38 mol/L = 0.33 mol SrCl2
Next, we can use the stoichiometry of the balanced equation to determine the number of moles of NaF required:
0.33 mol SrCl2 x (2 mol NaF / 1 mol SrCl2) = 0.66 mol NaF
Finally, we can calculate the volume of a 0.35 M NaF solution containing 0.66 moles of NaF:
0.66 mol NaF / 0.35 mol/L = 1.89 L or 1890 mL
Therefore, 1890 mL of a 0.35 M NaF solution would be needed to completely react with 869 mL of a 0.38 M SrCl2 solution.
To know more about stoichiometry refer here:
https://brainly.com/question/30215297
#SPJ11
Which of the following is a property of water?
Answers
0.82 moles of CH4, how many grams is it
Answers
Answer:
13.1548172
Explanation:
The SI base unit for amount of substance is the mole. 1 mole is equal to 1 moles CH4, or 16.04246 gram
so 0.82 × 16.04246
Pls consider Brainliest-ing my answer! It would mean a lot! ;)
36) What is the mass of a 4.259 g/cm substance which takes up 250.00 cm of space?
Answers
Answer:
The answer is 1064.75 gExplanation:
The mass of a substance when given the density and volume can be found by using the formula
mass = Density × volumeFrom the question
volume of substance = 250 cm³
density = 4.259 g/cm³
We have
mass = 4.259 × 250
We have the final answer as
1064.75 gHope this helps you
Will give brainliest
What is ozone depletion in simple words
Answers
Answer:
Ozone layer depletion is the thinning of the ozone layer present in the upper atmosphere. This happens when the chlorine and bromine atoms in the atmosphere come in contact with ozone and destroy the ozone molecules. One chlorine can destroy 100,000 molecules of ozone. It is destroyed more quickly than it is created
Answer:
Ozone depletion, gradual thinning of Earth’s ozone layer in the upper atmosphere caused by the release of chemical compounds containing gaseous chlorine or bromine from industry and other human activities. The thinning is most pronounced in the polar regions, especially over Antarctica. Ozone depletion is a major environmental problem because it increases the amount of ultraviolet (UV) radiation that reaches Earth’s surface, which increases the rate of skin cancer, eye cataracts, and genetic and immune system damage. The Montreal Protocol, ratified in 1987, was the first of several comprehensive international agreements enacted to halt the production and use of ozone-depleting chemicals. As a result of continued international cooperation on this issue, the ozone layer is expected to recover over time.
A gas made up of N and O contains 30.4% N. At STP (0 C and 1 atm), 4.0 g of the gas occupies a volume of 0.974 L. Calculate the molecular formula.
Answers
The molecular formula of the compound, given that the compound made up of N and O contains 30.4% N is N₂O₄
How do i determine the molecular formula of the compoundFirst, we shall obtain the molar mass of the compound. Details below:
Volume (V) = 0.974 LTemperature (T) = 0 °C = 0 + 273 = 273 KPressure (P) = 1 atmGas constant (R) = 0.0821 atm.L/mol KMass = 4.0 gMolar mass = ?The mole of the gas is obtained as follow:
PV = nRT
1 × 0.974 = n × 0.0821 × 273
Divide both sides by 24.0553
n = 0.974 / (0.0821 × 273)
n = 0.043 mole
Thus, the molar mass is obtained as:
Molar mass = mass / mole
Molar mass = 4 / 0.043
Molar mass = 93 g/mol
Next, we shall obtain the empirical formula of the compound. details below:
Nitrogen (N) = 30.4%Oxygen (O) = 100 - 30.4 = 69.6%Empirical formula =?Divide by their molar mass
N = 30.4 / 14 = 2.171
O = 69.6 / 16 = 4.35
Divide by the smallest
N = 2.171 / 2.171 = 1
O = 4.35 / 2.171 = 2
Thus, the empirical formula is NO₂
Finally, we shall obtain the molecular formula of the compound. This is shown below:
Empirical formula = NO₂Molar mass of compound = 93 g/molMolecular formula =?Molecular formula = empirical × n = mass number
[NO₂]n = 150
[14 + (2 × 16)]n = 150
46n = 93
Divide both sides by 46
n = 93 / 46
n = 2
Molecular formula = [NO₂]n
Molecular formula = [NO₂]₂
Molecular formula = N₂O₄
Thus, the molecular formula of the compound is N₂O₄
Learn more about molecular formula:
https://brainly.com/question/29096809
#SPJ4
Where is altitude measured in meters? How did this affect the MD-11?
Answers
Answer:
China, Mongolia, Russia, and North Korea. They were told to fly at 1400 meters but they flew at 1500 feet making them crash.
Explanation:
Identify the state of matter represented by each particle model.

Answers
A liquid
B solid
c gas
the internolecular forces in a solid state are packed and leave no spaces and in a liquid are semi packed and leave little space and in gas the particles are loose
a solid has ___ shape and ___ volume can anyone tell me the answer
Answers
Answer:
fixed shape and a definite volume
Explanation:
Answer:
a solid has a definite shape and definte volume
Explanation:
which type of change occurs when the electrons of two atoms interact to form a chemical bond?
Answers
Electrons are transferred from one atom to another.
Options:
Ionic Bond or Covalent Bond?
Answers
Answer:
ionic
Explanation:
ionic transfer of e^- ions formed (charges)
ionic=non-metal+ metal
ex: F+Ca
covalent sharing e^- no true charges
covalent= non-metal+ non-metal
ex: F+P
( my notes)
Write balanced equation of carbon burning in air
Answers
Answer:
When carbon is burned in air, it forms carbon dioxide gas and releases a large amount of heat and some light:
C+O2= CO2+ heat+ light
Answer:
When carbon is burned in air, it forms carbon dioxide gas and releases a large amount of heat and some light:
C+O2= CO2+ heat+ light
Explanation:
I need some help on this please! Find the expectation value of the radial distance of the electron for the 1s state of H. Show how you did the integral or state where you found it from.
Answers
E(r) = 8π * [(-r^3/a_0^3 + 3r^2/a_0^2 - 6r/a_0 + 6) * exp(-r/a_0)] This is the expression for the expectation value of the radial distance of the electron in the 1s state of hydrogen.
To find the expectation value of the radial distance of the electron for the 1s state of hydrogen (H), we need to calculate the integral of the radial distance multiplied by the probability density function over all space.
In the case of the 1s state of hydrogen, the radial wavefunction is given by:
R(r) = (2/a_0^3/2) * exp(-r/a_0)
Where a_0 is the Bohr radius.
To calculate the expectation value, we need to integrate the radial distance r multiplied by the square of the radial wavefunction |R(r)|^2 over all space.
E(r) = ∫ (r * |R(r)|^2) * 4πr^2 dr
Since the 1s orbital is spherically symmetric, we integrate over all space from 0 to infinity.
To simplify the calculation, we can substitute u = r/a_0:
E(u) = ∫ (u * |R(u * a_0)|^2) * 4π(a_0^3) du
Now, substituting the radial wavefunction R(u * a_0):
E(u) = ∫ (u * (2/a_0^3/2) * exp(-u)) * 4π(a_0^3) du
Simplifying further:
E(u) = 8π ∫ u^3 * exp(-u) du
This integral can be solved using integration by parts. Let's denote I(n) as the integral of u^n * exp(-u) du. We can then express I(n) in terms of I(n-1):
I(n) = -u^n * exp(-u) + n * I(n-1)
Using this relation, we can calculate E(u):
E(u) = 8π * [ -u^3 * exp(-u) + 3 * (-u^2 * exp(-u) + 2 * (-u * exp(-u) + I(0)))]
Simplifying further:
E(u) = 8π * [-u^3 * exp(-u) + 3 * (-u^2 * exp(-u) + 2 * (-u * exp(-u) + exp(-u)))]
E(u) = 8π * [(-u^3 + 3u^2 - 6u + 6) * exp(-u)]
Finally, substituting u = r/a_0 back into the equation, we obtain the expectation value of the radial distance for the 1s state of hydrogen:
E(r) = 8π * [(-r^3/a_0^3 + 3r^2/a_0^2 - 6r/a_0 + 6) * exp(-r/a_0)]
This is the expression for the expectation value of the radial distance of the electron in the 1s state of hydrogen.
To know more about radial wavefunction, visit:
https://brainly.com/question/30509565
#SPJ11
Why do certain Snurfles not survive?
Answers
IF U ANSWER THIS ILL BRAINLIST U N GIVE U 5 STAR. just answer the ones u know if ur a science genius or whateva. ahah ty.
1) what is the electron configuration of oxygen?
a) 2, 6
b) 2, 8, 8
c) 2, 8, 5
d) 2, 2, 2, 2
2) an atom contains 6 protons, u neutrons and 6 electrons. it's atomic number is?
a) 6
b) 7
c) 19
d) 13
3) which of the following statements about atoms is false?
a) an atom is mostly empty space.
b) neutrons and protons are found in the nucleus with the electrons.
c) the number of neutrons found in the nucleus of an atom is always the same.
d) electrons circle the neucleus all the time.
Answers
Answer:
a) 2, 6
a) 6
b) neutrons and protons are found in the nucleus with the electrons.
Explanation:
1.
Electronic configuration entails the arrangement of electrons within an atom. Therefore, to do this, we must know the number of electrons in the element given.
since we have been given oxygen atom, the number of electrons therein is 8.
So;
the electronic configuration is 2,6
2.
The atomic number of an atom is the same as the number of protons that it contains. The proton number is the number of positively charged particles in the atom of the element.
Since we have been given that the number of protons within the atom is 6, therefore, the atomic number is 6.
3.
It is false that the nucleus of an atom contains protons, neutrons and electrons. Electrons are not found in the nucleus of any atom. Only the process of electron capture during radioactivity can make this happen.
Electrons are found orbiting the atomic space in discrete energy levels.
What happens when there is an increase in temperature for a reaction rate? Select all that apply.

Answers
ANSWER
option A and B
EXPLANATION
An increase in temperature will increase the average kinetic energy of the molecule, thereby increasing the frequency of collision.
The correct answer are option A and B
Determine whether each statement is a description of a physical property or a chemical property. Paper burns readily in air. Physical chemical Salt is a solid. Physical chemical.
Answers
Answer:
paper burning is chemical property because burning is a chemical reaction. salt being a solid is a physical property because phases of matter are physical properties of matter
Answer:
answer in ppicture
Explanation:

if the velocity of an object is doubled, its kinetic energy is multiplied by