What is the "percent
abundance" of the cannellini
beans in this sample?
Sample Number Abundance Mass
of Beans
(%)
(g)
187
1.3
Black
Eyed Peas
Cannellini
Beans
113
[?]
Percent Abundance
2.9
Weighted
Average (g)
1.9
Enter

Answers
The "percent abundance" of the cannellini beans in this sample is 11.76 %.
What is percent ?In essence, percentages are fractions with a 100 as the denominator. We place the percent symbol (%) next to the number to indicate that the number is a percentage. For instance, you would have received a 75% grade if you answered 75 out of 100 questions correctly on a test (75/100).
The word percentage comes from the Latin term per centum, which means "by the hundred." In the 16th century, the Latin phrase made its way into English. Later, it was shortened to percent and ended with a period. Later, the period was eliminated, and the two portions were combined to create the current one-word form of percent.
Thus, The "percent abundance" of the cannellini beans in this sample is 11.76 %.
To learn more about the percent, follow the link;
https://brainly.com/question/14699403
#SPJ1
Related Questions
nitrogen monoxide and oxygen react to form nitrogen dioxide. if 5.6 moles of no react with 3.1 moles of o 2, how many moles of the reactant in excess will remain after the reaction? (assume 100% yield.)
Answers
0.3 moles of Oxygen is excess reactant (reagent) when yield is 100% .
NO =5.6 moles
O2= 3.1 moles
2NO + O2 --> 2NO2 the chemical reaction.
the ratio of NO and O2 is 2:1
therefore for 5.6 moles of NO, 2.8 moles of Oxygen(O2) is required. So 3.1moles - 2.8 moles = 0.3 moles
The reactant in a chemical reaction that is present in a quantity that is larger than what is required to fully react with the limiting yield is known as the excess reactant. The substance(s) still present after a chemical reaction has reached equilibrium in yield are the reactant(s). Subtract the mass of excess reagent eaten from the total mass of excess reagent provided to determine the quantity of yield that is still present. Reactants in a chemical reaction are referred to as excess reagents if they are still present after the reaction is complete.
learn more about yield here:
https://brainly.com/question/25996347
#SPJ4
which procedure is recommended when a student needs more of a hazardous material
Answers
When a student needs more of a hazardous material, the recommended procedure is to follow proper safety protocols and guidelines, including consulting with the instructor or supervisor.
Handling hazardous materials requires careful consideration of safety measures to minimize the risks involved. In this situation, the student should first consult with their instructor or supervisor to communicate their need for more of the hazardous material. The instructor or supervisor can provide guidance on the appropriate procedures to follow, including assessing the necessity for the material and ensuring that the student has the necessary training and knowledge to handle it safely.
It is crucial to understand the risks associated with the hazardous material and follow all safety guidelines and protocols. This may include wearing appropriate personal protective equipment (PPE), working in a well-ventilated area, and using proper storage and disposal methods.
Furthermore, it is important to adhere to any legal requirements or regulations regarding the procurement and handling of hazardous materials. This may involve obtaining the necessary permits or licenses, ensuring compliance with safety standards, and maintaining proper documentation.
By following these recommended procedures, students can ensure their safety and the safety of others while obtaining the required amount of hazardous materials.
Learn more about hazardous material here: brainly.com/question/30264140
#SPJ11
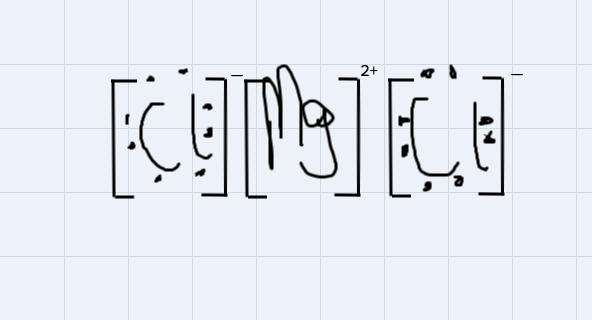
Use Lewis symbols to show how MgCl2 will be formed from Mg and Cl2.
Answers
This is a type of bonding that is formed from the from the attraction of oppositely charged ions in a compound.
For instance, MgCl2 is an ionic compound because the 2 positive ions wipossessed by the magnessium atom will attract each of the negtaive ion possessed by each of the chlorine atom to form the magnessium chloride compound
Using the Lewis symbol to demonstrate the bondng:
From the disgram, the negative ions on chlorine atoms will get attracted to the positive ions on the magnessium ion.
a 5.08 g mixture contains both lithium fluoride, lif, and potassium fluoride, kf. if the mixture contains 3.26 g fluorine, what is the mass of the kf in the mixture?
Answers
The mass of KF in the mixture is 1.067 grams
As per the details provided in the question are as follow,
Total weight of mixture (LiF+KF)= 5.08 grams
Mixture contains 3.26 grams of fluorine
Molar mass K = 39.10 g/mol
Molar mass F = 19.00 g/mol
Molar mass KF = 58.10 g/mol
Molar mass of lithium = 6.94 g/mol
mass of KF and LiF
Mass KF = X grams
Mass LiF = 5.08 -X grams
Now Calculate the moles
Moles KF = x grams / 58.10 g/mol
Moles LiF = (5.08-X grams) / 25.94 g/mol
1 mol of KF contains 1 mol of F atoms.
1 mol of LiF contains 1 mol of F atoms.
moles of F in KF= moles of KF= x/58.10 g/mol
moles of F in LiF = moles of LiF= (5.08-x)/25.94g/mol
Total moles of Fluorine =(x/58.10)+((5.08-x)/25.94)
Calculate mass
The total weight of Fluorine in sample
3.26 grams =((x/58.10)+((5.08-x)/25.94))*19g/mol
x = 1.067 grams
For such more question on mass .
https://brainly.com/question/19385703
#SPJ4
Which of the follow can NOT be possible
error that occurs during a lab experiment?
human error
pure reactants were used
thing
O unexpected products were produced
the reaction didn't go to completion
Answers
Answer:did h find the answer
Explanation:
What is the empirical formula of compund that contains 4. 03g of hydrogen per mole, 64. 14 g of sulfur per mole and 128 g of oxyen per mole
Answers
The empirical formula of compound that contains 4. 03g of hydrogen per mole, 64. 14 g of sulfur per mole and 128 g of oxygen per mole is H₂SO₂.
given that :
mass of hydrogen = 4.03 g
mass of sulfur = 64.14 g
mass of oxygen = 128 g
moles of hydrogen = mass / molar mass
= 4.03 / 1
= 4mol
moles of sulfur = 64.14 / 32
= 2 mol
moles of oxygen = 128 / 32
= 4 mol
dividing by the smallest one , we get
mole of H = 2
mole of S = 1
mole of O = 2
The empirical formula = H₂SO₂
Thus, the empirical formula of compound that contains 4. 03g of hydrogen per mole, 64. 14 g of sulfur per mole and 128 g of oxygen per mole is H₂SO₂.
To learn more about empirical formula here
https://brainly.com/question/25780603
#SPJ4
suppose that a system A is placed into thermal contact with a heat reservoir A' which is at an absolute temperature T' and that A absorbs amount of heat Q in the process. Show that the entropy increases delta S of A in this process satisfies the inequality deltaS>=Q' T ' , where the equal sign is valid if the initial temperature of A differs infinitesimally from the temperatutre T' of A' . culled from statistical and thermal Physics by F. Reif question 3.4
Answers
When system A absorbs heat Q from reservoir A' at temperature T', the entropy change of system A can be expressed as: ΔS = Q/T
where T is the initial temperature of system A before absorbing heat. To show that ΔS ≥ Q' T', we can use the fact that the entropy of a system can never decrease spontaneously, i.e., ΔS ≥ 0. Therefore, ΔS = Q/T ≥ 0 Multiplying both sides by T', we get: Q' T' = T'ΔS = T'(Q/T) = QT'/T ≥ 0. Thus, we have shown that ΔS ≥ Q' T', with equality if and only if T is infinitesimally close to T'. This result is a fundamental principle of thermodynamics, known as the Second Law of Thermodynamics, which states that the entropy of a closed system can never decrease over time, but can only increase or remain constant.
To learn morte about Thermodynamics click thje link below
brainly.com/question/1368306
#SPJ4
Pls I need help urgently. What can be predicted about meniscus formation if the adhesion and cohesion forces were equal in the graduated cylinder?
Answers
If the adhesion and cohesion forces were equal in a graduated cylinder, there would be no meniscus formation. This is because the shape of a meniscus is a result of the difference in strength between the adhesive and cohesive forces between the liquid and the surface it comes into contact with.
If the adhesive forces were equal to the cohesive forces, the liquid would not be attracted more strongly to the surface of the container than to its own molecules. As a result, the surface of the liquid would be flat and level with the edge of the container. This is the case with mercury, which has approximately equal cohesive and adhesive forces, resulting in a flat surface in a container.
However, in most cases, the adhesive forces between a liquid and a container are stronger than the cohesive forces between the liquid molecules themselves, resulting in a concave meniscus. This is because the liquid is attracted more strongly to the surface of the container than to its own molecules, causing the surface of the liquid to curve downward at the edges. On the other hand, if the cohesive forces were stronger than the adhesive forces, the liquid would form a convex meniscus, with the surface of the liquid curving upward at the edges. This is the case with water in a hydrophobic container, such as a wax-coated or greasy container.
BRAINLIEST Which of the following images is drawn incorrectly? (Picture)

Answers
Answer:
all are correct
Explanation:
AI Consist of 3 valence electrons we would make all tree images correct
i gave brainlest.... the answer is correct!!!

Answers
1.Oort Cloud
2.Uranus Earth and Mars
3.Kuiper Belt
4.Asteroid Belt
2.Uranus Earth and Mars
3.Kuiper Belt
4.Asteroid Belt
Emily is a competitive swimmer. Why does wearing a swim cap help Emily swim faster?
A) The cap reduces her inertia.
B) The cap keeps the hair out of her eyes.
C) The cap increases the friction between her and the water.
D) The cap decreases the friction between her and the water.
Answers
the answer is
B) the cap keeps the hair out of her eyes
I SWEAR IF IM WRONG THAN EMILY CAPPING CUZ MY CAP DECTOR IS GOING OFF
True or False: Polypeptides inside the ER are usually smaller than polypeptides synthesized from the same mRNA that have not entered the ER.
Answers
True. For these before leaving the ER undergo a process called N-terminal signal peptide cleavage. During this process, the signal peptide, which is an amino acid sequence that helps guide the protein into the ER, is cleaved, resulting in a smaller polypeptide.
What are Polypeptides?Polypeptides are composed of a variety of different amino acids that are linked together in a sequence. This sequence of amino acids determines the structure and function of the protein that they make up. Polypeptides are essential components of cells, playing a role in many essential processes, including:
MetabolismSignalingGene expressionThey are found in the cytoplasm and nucleus of cells, as well as in extracellular fluid. Polypeptides are also important components of:
HormonesEnzymesAntibodiesLearn more about The Polypeptides:
https://brainly.com/question/14183859
#SPJ4
Shielding effect increases top to bottom in a group because
Answers
Answer:
The number of energy level increases
Explanation:
Shielding effect increases from top to bottom in a group because the number of energy level increases.
Shielding effect is the process by which electrons in an atom protects one another from the pulling effect of the nucleus.
Down a group, the shielding effect increases due to increased number of electronic shell.
The probability that it is friday and your day off is 12%. What is the probability that it is your day off given that today is friday?.
Answers
The probability that it is your day off given that today is friday 12% or 0.12. Conditional probability is the probability of an event occurring given that another event has already occurred.
We can use Bayes' Theorem, which states that:
P(A | B) = P(B | A) * P(A) / P(B)
Where:
P(A) is the probability of event A happening (it is your day off)
P(B) is the probability of event B happening (today is Friday)
P(A | B) is the probability of event A happening, given that event B has happened (the probability that it is your day off, given that today is Friday)
P(B | A) is the probability of event B happening, given that event A has happened (the probability that it is Friday, given that it is your day off)
We know that the probability of it being Friday and your day off is 12%. This is the joint probability of events A and B.
P(A and B) = P(A) * P(B) = 0.12
We are also given that the probability that it is Friday and it's your day off is 12% which is equivalent to P(A and B)
We don't have the information about the probability of B given A, but we have P(A and B) and we know the probability of B and A.
so we can calculate P(A|B) as follows:
P(A|B) = P(A and B) / P(B) = (0.12) / (0.12 + x)
Where x is the probability of B given A is not true.
This formula tells us that the probability that it is your day off, given that today is Friday, is 12% or 0.12
Learn more about Probability here:
https://brainly.com/question/30034780
#SPJ4
Given that it is Friday today, the likelihood that it is your day off is 12%, or 0.12. The likelihood of an event happening given that another event has already happened is known as conditional probability.
Using the Bayes' Theorem, which says:
P(B | A) * P(A) / P(A | B) = P(B | A) (B)
Where:
The probability of an event is P(A). an occurrence (it is your day off). P(B) is the likelihood that event B will occur (today is Friday)
P(A | B) represents the likelihood that event A will occur given that event B has already occurred (the probability that it is your day off, given that today is Friday). P(B | A) is the likelihood that event B will occur assuming that event A has already occurred (the likelihood that it will be Friday).
To learn more about probability, click here.
https://brainly.com/question/30034780
#SPJ4
monomer of nucleic acids made up of a 5-carbon sugar, a phosphate group, and a nitrogenous base
Answers
Giant macromolecules known as nucleic acids are composed of nucleotide monomers. Pentose sugar, phosphate group, and nitrogenous base are the three elements that make up nucleotides.
Is DNA or RNA made of phosphate sugar and bases?The nucleotides in a DNA sequence are joined by an alternating grey-dark grey sugar-phosphate backbone. The structural structure of nucleic acids, including DNA and RNA, is composed of the sugar-phosphate backbone. This molecule's directionality is determined by this backbone, which is made up of alternating sugar and phosphate groups.
What are the bases and sugars in RNA?However, RNA is often a single-stranded molecule, unlike DNA. Additionally, the name of the molecule is explained by the fact that ribose, rather than deoxyribose, is the sugar present in RNA. The four nitrogenous bases that make up RNA are adenine, cytosine, uracil and guanine.
To learn more about nucleic acid visit:
brainly.com/question/11309892
#SPJ4
The complete question is: What are monomer of nucleic acids made up of a 5-carbon sugar, a phosphate group, and a nitrogenous base called?
Write the formula for Zn+2 (SO4)-2
Answers
Answer:
Zn2 + SO4 = Zn2(SO4)2
Explanation:
Which notation represents the largest atomic radius?
Cl
Cl^−
F
F^−
Answers
Answer:
Cl⁻ Or A
Explanation:
The equation below shows hydrogen reacting with oxygen to produce water.
2H2 + O2 Right arrow. 2H2O
If 16 mol of oxygen were reacted with excess hydrogen gas, how many moles of water would be produced?
4.0 mol
8.0 mol
16 mol
32 mol
Answers
The equation below shows hydrogen reacting with oxygen to produce water 2H₂ + O₂ → 2H₂O If 16 mole of oxygen were reacted with excess hydrogen gas, then 32 mole of water would be produced
Mole is the amount of substance of a system which contains as many elementary entities
Here given data is
Hydrogen reacting with oxygen to produce water and the reaction is
2H₂ + O₂ → 2H₂O
16 mol of oxygen were reacted with excess hydrogen gas
Then we have to calculate moles of water would be produced = ?
Moles of oxygen = 16 moles
From the balanced equation
1 mole oxygen react to give 2 moles of water
16 moles oxygen react to give 2×16 = 32 moles
Therefore 32 moles of water would be produced in the reaction
Know more about mole
https://brainly.com/question/10811423
#SPJ1
d. 32 mol in case you dont want to read that
What is the limiting reactant in the balloon with 7.5g baking soda and vinegar?
Answers
In the last flask, which contained 7.5g of baking soda initially, add vinegar. The fact that a reaction takes place proves that vinegar was the limiting reactant.
What limiting reagent is used in the balloon experiment?
Each flask should contain about 15 drops of bromothymol blue after the balloons have been removed. The first flask, which contained 2.0 baking soda initially, should now contain baking soda. The fact that a reaction takes place proves that baking soda was the limiting reactant. Calculating how much product each reactant can produce and identifying the limiting reagent by which one produces the least amount of product is one method of doing so.
Since oxygen is the limiting reactant, lowering the oxygen input is the best way to reduce soot.
To learn more about oxygen refer to:
brainly.com/question/26073928
#SPJ1
for this investigation, you used commercial juices. the juices are known to contain appreciable amounts of malic acid. the malic acid in the juice sample will [ select ] . this means the volume of [ select ] used in the neutralization reaction is [ select ] what it should have been, making it appear like there are [ select ] present. therefore, the calculated concentration of the [ select ] in the juice samples will be [ select ] the actual concentration in the commercial juices.
Answers
For this investigation, you used commercial juices, which are known to contain appreciable amounts of malic acid. The malic acid in the juice sample will undergo neutralization.
This means the volume of base used in the neutralization reaction is less than what it should have been, making it appear like there are more moles of malic acid present.
Therefore, the calculated concentration of the malic acid in the juice samples will be greater than the actual concentration in the commercial juices.
This means the volume of NaOH used in the neutralization reaction is less than what it should have been, making it appear like there are more malic acids present.
What is neutralization? Neutralization is the chemical reaction between an acid and a base to produce a salt and water.
This chemical reaction takes place between hydrogen ions (H+) and hydroxide ions (OH-).
The main aim of neutralization is to balance the acid and base's pH level.
When the pH value is around 7, it means that the acid and base are neutralized.
For more information about neutralization refer here
https://brainly.com/question/15347368
#SPJ11
trans-1,4-dibromocylohexane is more stable than the cis isomer because only the trans isomer can adopt a conformation in which both bromine atoms are in orientations.
Answers
It's true that, the trans isomer of trans-1,4-dibromocylohexane is more stable than the cis isomer because the trans isomer can adopt a conformation in which both bromine atoms are in orientations, while the cis isomer cannot. This conformation gives the trans isomer a more stable structure, making it more stable than the cis isomer.
The statement "trans-1,4-dibromocylohexane is more stable than the cis isomer because only the trans isomer can adopt a conformation in which both bromine atoms are in orientations" is correct. The trans isomer of 1,4-dibromocylohexane is more stable than the cis isomer because the trans isomer can adopt a chair conformation in which both bromine atoms are in equatorial positions. This allows for the bromine atoms to be as far apart from each other as possible, reducing steric strain and increasing stability. In contrast, the cis isomer cannot adopt a conformation in which both bromine atoms are in equatorial positions, leading to increased steric strain and decreased stability. Therefore, the trans isomer of 1,4-dibromocylohexane is more stable than the cis isomer.
Your question is incomplete but most probably your full question was
Trans-1,4-dibromocylohexane is more stable than the cis isomer because only the trans isomer can adopt a conformation in which both bromine atoms are in orientations. True or false.
Learn more about isomers at
https://brainly.com/question/30330846
#SPJ11
Base your answer on the information and illustrations below and on your knowledge of biology. The illustrations represent cross sections of two different plant stems.
A student compared two stem cross sections. Stem cross section A is from a plant that can be used to produce products with valuable medicinal properties. Stem cross section B is from a plant growing in the same area of the forest and its usefulness for producing medicines is unknown. The student concluded that the stem cross sections had many structural similarities and that the plant that produced cross section B would produce the same valuable medicinal products.
Is the student's conclusion valid?
A) Yes, because the structural similarities indicate a close relationship between the organisms.
B) Yes, because these plants grow in the same regions of the forest ecosystem and look similar.
C) No, because he did not evaluate soil conditions, such as pH, with chemical indicators.
D) No, because this structural evidence alone is insufficient and molecular evidence should be obtained.

Answers
Option D is the correct answer. This is because the production of medicinal compounds is determined by the plant's genetics and biochemistry, which may not be reflected in the plant's structural features alone.
What is the students conclusion?The student's conclusion is not valid. While the two stem cross sections may have many structural similarities, this is not sufficient evidence to conclude that the plant that produced cross section B will produce the same valuable medicinal products as the plant that produced cross section A.
Option A and B are incorrect because structural similarities do not necessarily indicate a close relationship between organisms or their biochemical properties. Option C is also incorrect because while soil conditions may affect plant growth, they do not necessarily determine a plant's ability to produce specific medicinal compounds.
Learn more about stem cross sections:https://brainly.com/question/1653214
#SPJ1
Heavier noble gases are able to form compounds with other elements under specific conditions because their valence ____are farther from the _____
Answers
Answer:
Heavier noble gases are able to form compounds with other elements under specific conditions because their valence electrons are farther from the nucleus.
Explanation:
The name of noble or inert gas is due to the lack of reactivity with other elements. This is due to its electronic configuration, because its outermost shell or valence shell is always complete, without the need to share, give or receive electrons forming bonds. That is, its outer layer is so stable that the element tends not to react with others except in very specific cases.
These exceptions generally involve the heavier noble gases, such as xenon or radon, capable of forming compounds with fluorine and oxygen. This is because the heavier noble gases have more electron shells than the lighter ones. This characteristic causes the outermost electrons to experience a "shielding" effect due to the action of the inner electrons, and they can then be ionized more easily, since the attraction they receive from the positive charges of the nucleus is weaker. That makes the ionization energy low enough to form stable compounds with more electronegative elements, such as fluorine and oxygen.
Heavier noble gases are able to form compounds with other elements under specific conditions because their valence electrons are farther from the nucleus.
automobile batteries use 3.0 m h2so4 as an electrolyte. how much 1.20 m naoh will be needed to neutralize 225 ml of battery acid?
Answers
The amount of 1.20 m NaOH that will be needed to neutralize 225 ml of battery acid is 1125 ml.
The balanced chemical reaction is given as,
H₂SO₄ (aq) + 2 NaOh (aq) → 2 H₂O + Na₂SO₄ (aq)
Generally molarity is defined as one of the most widely used unit of concentration and it is denoted by M.
By formula of molarity,
V1M1 n2 = V2M2n1
V= volume
M = concentration in mole per liter
n = number of moles
V1 =?
V2 = 225 ml
M1 = 1.2 M
M2 = 3 m
n1 =2 moles
V1 is therefore = ( 225 x3 x2 ) /1.2 = 1125 ml
Learn more about molarity from the link given below.
https://brainly.com/question/31545539
#SPJ4
Tartaric acid is found in many fruits, including grapes, and is partially responsible for the dry texture of certain wines. Calculate the pH and the tartrate ion 1C4H4O6 2-2 concentration for a 0.250 M solution of tartaric acid, for which the acid-dissociation constants. Did you have to make any approximations or assumptions in your calculation?
Answers
No approximations or assumptions need to be made in the calculation of the pH and the tartrate ion 1C4H4O6 2-2 concentration for a 0.250 M solution of tartaric acid.
The pH and the tartrate ion 1C4H4O6 2-2 concentration of a 0.250 M solution of tartaric acid can be calculated using the acid-dissociation constants and the Henderson-Hasselbalch equation. The acid-dissociation constants (Ka1 and Ka2) of tartaric acid are 1.14x10-2 and 5.01x10-5, respectively.
The pH of the solution can be calculated using the Henderson-Hasselbalch equation: pH = pKa + log([base]/[acid]) where [base] is the concentration of the conjugate base (the tartrate ion) and [acid] is the concentration of the acid (tartaric acid). Since the solution is 0.250 M in tartaric acid, [acid] = 0.250 M and [base] = 0.250 M - [tartrate ion], which can be calculated using the Ka1 and Ka2 values.
For Ka1, the tartrate ion 1C4H4O6 2-2 concentration can be calculated as 0.250 M - 0.250 * 1.14x10-2 = 0.249 M. For Ka2, the tartrate ion 1C4H4O6 2-2 concentration can be calculated as 0.250 M - 0.250 * 5.01x10-5 = 0.249 M.
Using the Henderson-Hasselbalch equation, the pH of the solution can be calculated as pH = pKa + log([base]/[acid]). The pKa values of tartaric acid are 3.92 and 5.63 respectively. Therefore, for Ka1, the pH of the solution can be calculated as pH = 3.92 + log(0.249/0.250) = 3.91, and for Ka2, the pH of the solution can be calculated as pH = 5.63 + log(0.249/0.250) = 5.63.
No approximations or assumptions need to be made in the calculation of the pH and the tartrate ion 1C4H4O6 2-2 concentration for a 0.250 M solution of tartaric acid, as the Henderson-Hasselbalch equation and the acid-dissociation constants of tartaric acid can be used.
Learn more about Tartaric acid: brainly.com/question/24178503
#SPJ11
why are large atoms more reactive than small atoms
Answers
Answer:
large atoms have Valence electrons further from the nucleus and lose them more readily.
how to find bond order
Answers
To find the bond order of a molecule, subtract the number of bonding electrons from the number of anti-bonding electrons, and divide by 2.
The bond order is a measure of the strength and stability of a bond between two atoms in a molecule. It is calculated by determining the difference between the number of bonding electrons (those involved in forming a covalent bond) and anti-bonding electrons (those that weaken the bond) and dividing by 2.
The resulting number can range from zero to three, where higher bond orders indicate stronger and more stable bonds. A bond order of 0 indicates that the atoms do not form a bond, while a bond order of 1, 2, or 3 indicates a single, double, or triple bond, respectively. Bond order is an important concept in chemistry, as it helps predict the reactivity and behavior of molecules.
To know more about the bond order, here
brainly.com/question/12447843
#SPJ4
About how much of a star's life is spent as a main-sequence star?
Answers
Eighty Billion Years
What is the amount of mass in each given unit of volume?
Answers
Answer:
Each volumn is different and therefore has a specific and unique mass.
There are three sets of sketches below, showing the same pure molecular compound (hydrogen chloride, molecular formula ) at three different temperatures. The sketches are drawn as if a sample of hydrogen chloride were under a microscope so powerful that individual atoms could be seen. Only one sketch in each set is correct. Use the slider to choose the correct sketch in each set. You may need the following information: melting point of : -114.8 boiling point of : -85.1
Answers
Answer:
The correct answer is - option C.
Explanation:
Given: the melting point of HCl is
-114.8 °C, which suggests that below this temperature HCl will be solid.
and, since the boiling point of HCl is - 85.1 °C. It is also suggested that above this temperature HCl will be gas, Therefore.
Solid -114.8 - Ordered arrangement
Liquid -85.1c - Less orderly arranged
Gaseous - Least orderly arranged
Thus, at —90 °C, HCl will be present 'in the liquid state, At — 1 °C, HCl will be present in the gaseous state and at -129 °C, HCl will be present in the solid-state. So, the molecules will be organized in a more orderly manner .
Thus, the correct answer is - option C
