what is the molecular formula of a compound that contains 38% c, 45% n and 16% h if 0.157 g of the compound occupies 125 ml with a pressure
Answers
The molecular formula of the given compound is CH₅N.
What is the ideal gas equation?The ideal gas law has defined the product of the volume and pressure as equal to the product of the gas constant (R) and absolute temperature of the gas.
The mathematical equation of the ideal gas law is as follows:
PV = nRT
The temperature of the compound, T = 22° C = 295 K
The pressure of the compound, P = 99.5 KPa = 0.982 atm
The volume of the compound, V = 125 ml = 0.125 L
0.125 × 0.982 = (0.157 g/M) × 0.082 ×295
M = 31 g/mol
A number of moles of C = 0.157 ×0.37/12 = 0.00484
A number of moles of N = 0.157 ×0.45/14 = 0.00504
A number of moles of H = 0.157 ×0.16/1 = 0.0251
The simplest ratio of C: N: H is 1 : 1 : 5
The empirical formula of the given compound is CH₅N.
The molecular formula of the compound is CH₅N as the molecular mass is equal to 31 g/mol.
Learn more about ideal gas equation, here:
brainly.com/question/3637553
#SPJ1
Related Questions
I need the definition of Friction, choose one! Thank you

Answers
Answer:
the force resisting motion. it slows things down
what charge would bromine obtained when it becomes an ion
Answers
When bromine becomes an ion, it obtains a negative charge. The reason behind this is that Bromine has a tendency to accept an electron to complete its octet, which results in the formation of the Br- ion.
The electronic configuration of Bromine is 2, 8, 18, 7Here, we see that Bromine has seven valence electrons. The tendency to complete the octet leads to the acceptance of one electron, which makes the Bromine ion have an electronic configuration of 2, 8, 18.
This configuration is similar to the nearest noble gas, Krypton. We can conclude that the Bromine ion has a negative charge.
Know more about bromine:
https://brainly.com/question/29557040
#SPJ11
Bromine becomes a bromide ion with a charge of -1 when it gains an electron to fulfill the octet rule.
When bromine becomes an ion, it generally gains an electron to have a full outer electron shell in accordance with the octet rule. This gain of one electron increases its net charge by one negative unit, resulting in a bromide ion with a charge of -1. Keep in mind this is because halogen elements like bromine often become ions (anions specifically) by gaining an electron, not losing one.
Learn more about Ion here:https://brainly.com/question/31846310
#SPJ12
what is ionic compounds??
Answers
Answer:
The ionic compounds are chemical compounds composed of ions, which is held together by electrostatic forces termed ionic bonding.
Molecular systems tend to move spontaneously to a state of maximum randomness or disorder. Molecular randomness, or disorder, is called entropy and is denoted by the symbol S. As a state function, entropy change, ?S, depends only on initial and final states. ?S has a positive value when disorder increases and a negative value when disorder decreases. The following conditions usually result in an increase in entropy:
a change of phase: solid?liquid?gas,
an increase in the number of gas molecules, or
a solid dissolving to form a solution.
Although the sign of the entropy change can be predicted as described above, the actual value of ?S? must be calculated from the absolute entropy values, S?, of the reactants and products:
?S?=S?(products)?S?(reactants)
Answers
The change in entropy, denoted as ΔS, is a measure of the randomness or disorder of a system, and it depends on the initial and final states of the system.
A positive value of ΔS indicates an increase in randomness or disorder, while a negative value indicates a decrease in randomness or disorder.
There are several common scenarios where entropy tends to increase:
Change of phase: When a substance changes from a solid to a liquid or from a liquid to a gas, the entropy generally increases. This is because the particles in the substance have more freedom of movement in the liquid or gas phase, resulting in a higher degree of randomness.
Increase in the number of gas molecules: When the number of gas molecules increases, the entropy generally increases. This is because gas molecules are more randomly distributed and have greater freedom of movement compared to molecules in a condensed phase, such as a solid or liquid.
Dissolution of a solid to form a solution: When a solid dissolves in a solvent to form a solution, the entropy generally increases. This is because the particles in the solid become more dispersed in the solution, resulting in a higher degree of randomness.
To calculate the actual change in entropy, ΔS, for a given reaction, the absolute entropy values, denoted as S, of the reactants and products must be considered.
The change in entropy, ΔS, is then given by the difference between the entropy of the products and the entropy of the reactants, as expressed in the equation ΔS = S(products) - S(reactants).
Learn more about “ randomness or disorder “ visit here;
https://brainly.com/question/29128278
#SPJ4
Glucose (C6H12O6) and altrose (C6H12O6) can form glycosidic bonds to create polysaccharides. What is the chemical formula of a polymer made from 4 glucose and 2 altrose
Answers
Polysaccharides are formed by bonding monosaccharides through a glycosidic bond. This is what allows us to obtain long chains of monosaccharides that form the structural support for the cell walls of plants and animals. To determine the chemical formula of a polymer made from 4 glucose and 2 altroses,
we will first write down the molecular formula of each monosaccharide. Glucose: C6H12O6Altrose: C6H12O6A polymer of 4 glucose monosaccharides would have the chemical formula: 4(C6H12O6) = C24H42O21A polymer of 2 altrose monosaccharides would have the chemical formula: 2(C6H12O6) = C12H22O11To determine the chemical formula of a polymer made from 4 glucose and 2 altroses,
we need to add the two chemical formulas together: C24H42O21 + C12H22O11 = C36H64O32Therefore, the chemical formula of a polymer made from 4 glucose and 2 altroses is C36H64O32.
to know more about Glucose here:
brainly.com/question/13555266
#SPJ11
The answer is not 53.4. Please help.

Answers
Answer:
it is 0.528 ATM.
Explanation:
What are some examples of HIGH resistance? Question 2 options:
long wire, wide wire, more resistors
wide wire, short wire, less resistors
long wire, narrow wire, more resistors
narrow wire, less resistors, long wire
(PLEASE HURRY! I NEED TO FINISH THIS QUIZ)
Answers
Answer:
long wire, wide wire, more resistors
long wire, narrow wire, more resistors
Explanation:
longer wires have greater resistance. The longer the wire the greater the resistance.
The answer is Long wire, Narrow wire, more resistors
Evidence:

What is the latent heat of vaporization of boiling water?
a. 955 Btu/lb
b. 540 cal/gm
c. 2557 Kj/kg
d. 144 Btu/lb
Answers
The latent heat of vaporization of boiling water is 540 cal/gm. Option b is the correct answer.
It is the amount of heat needed to convert 1 gram of water from liquid to vapor state at atmospheric pressure at a constant temperature. The latent heat of vaporization for water is relatively high compared to other liquids because of the strong hydrogen bonding between water molecules. This means that water requires a lot of energy to break these bonds and change from a liquid to a gaseous state. The latent heat of vaporization is also responsible for the cooling effect of evaporation. When sweat evaporates from our skin, it absorbs heat from our body, which helps to cool us down. In conclusion, the latent heat of vaporization of boiling water is 540 cal/gm, which represents the amount of heat required to convert 1 gram of water from liquid to vapor state at atmospheric pressure at a constant temperature.
know more about latent heat
https://brainly.com/question/23976436
#SPJ11
If a screwdriver was used to pry open a door, where would scratches/striations be found?
Answers
Answer: Most likely around the knob area or by the bolts that hold the door up.
Explanation:
If you attempted to pry open a locked window with a screwdriver, the screwdriver would leave a tool mark on the window and windowsill.
The impressions made by a tool when it comes in contact with an object are called as tool marks. These tool marks exhibit a variety of impressions depending upon the type of tool, its shape and its use. They are classified into different types depending upon the force with which they are made.
The study of tool marks is very useful in forensic science which includes information about the analysis of tool marks at the crime scene and in the laboratory, the interpretation and assessment of challenges for examination and interpretation and also the way in which tool mark evidence can be presented in a courtroom.
Learn more about Tool marks, here:
https://brainly.com/question/32257286
#SPJ2
In dry climates, people often dig wells to find additional sources of water
to raise crops or feed livestock. Which of the following could be
significant negative effect on the environment cause by obtaining water
from the well?
Select one:
A: The digging will create air pockets in the soil.
B: Using the well could erode the soil and increased the risk for mountain
landslides.
C: Crushed rock and layers of soil might pile up where the well is dug
D: Using the well could lower the water table throughout the area.
Answers
Answer:
D i think, sorry if its wrong
Explanation:
Answer:
Using the well could lower the water table throughout the area.
Explanation:
i just took a test and this was the answer
In an experiment, 3.25 g of C3H8 react with 3.50 g of O2.1) Write the formula for the reactant that is the limiting reactant.
Answers
So,
The reaction that takes place here is the next one:
The first thing we're going to do is to pass the mass of each compound to moles. (We do this just dividing by the molecular weight of the compound):
Now, the last step is just to divide each amount by the coefficient of the reaction of each compound. The smaller result will be the limiting reactant.
Therefore, the limiting reactant is O2.
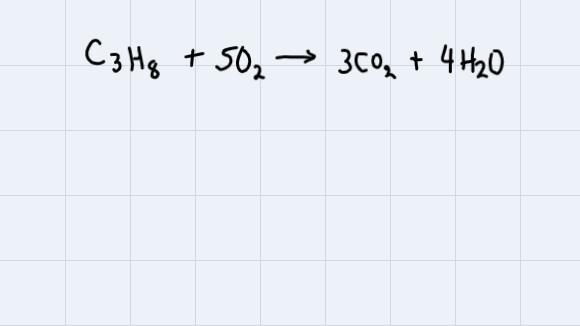


A 17.50-ml sample of a 0.150 m solution of h3po4 reacts with excess ba(oh)2. what mass of h2o is produced in the reaction?
Answers
Taking into account the reaction stoichiometry, 0.14175 grams of H₂O are formed when a 17.50 mL sample of a 0.150 M solution of H₃PO₄ reacts with excess Ba(OH)₂.
Reaction stoichiometryIn first place, the balanced reaction is:
2 H₃PO₄ + 3 Ba(OH)₂ → Ba₃(PO₄)₂ + 6 H₂O
By reaction stoichiometry (that is, the relationship between the amount of reagents and products in a chemical reaction), the following amounts of moles of each compound participate in the reaction:
H₃PO₄: 2 molesBa(OH)₂: 3 molesBa₃(PO₄)₂: 1 moleH₂O: 6 molesThe molar mass of the compounds is:
H₃PO₄: 98 g/moleBa(OH)₂: 171.34 g/moleBa₃(PO₄)₂: 602 g/moleH₂O: 18 g/moleThen, by reaction stoichiometry, the following mass quantities of each compound participate in the reaction:
H₃PO₄: 2 moles ×98 g/mole= 196 gramsBa(OH)₂: 3 moles ×171.34 g/mole= 514.02 gramsBa₃(PO₄)₂: 1 mole ×602 g/mole= 602 gramsH₂O: 6 moles ×18 g/mole= 108 gramsMoles of H₃PO₄ requiredMolar concentration or molarity is a measure of the concentration of a solute in a solution and indicates the number of moles of solute that are dissolved in a given volume.
The molarity of a solution is calculated by dividing the moles of solute by the volume of the solution:
molarity= amount of moles÷ volume
In this case, you know for the reactant H₃PO₄:
molarity= 0.150 Mvolume= 17.50 mL= 0.0175 L (being 1000 mL= 1 L)Replacing in the definition of molarity:
0.150 M= amount of moles÷ 0.0175 L
0.150 M× 0.0175 L= amount of moles
0.002625 moles= amount of moles
So, 0.002625 moles of H₃PO₄ react.
Mass of H₂O formedThe following rules of three can be applied: if by reaction stoichiometry 2 moles of H₃PO₄ form 108 grams of H₂O, 0.002625 H₃PO₄ form how much mass of H₂O?
\(mass of H_{2}O=\frac{0.002625 moles of H_{3}PO_{4} x108 grams of H_{2}O}{2moles of H_{3}PO_{4}}\)
mass of H₂O= 0.14175 grams
Finally, 0.14175 grams of H₂O are formed when a 17.50 mL sample of a 0.150 M solution of H₃PO₄ reacts with excess Ba(OH)₂.
Learn more about the reaction stoichiometry:
brainly.com/question/24741074
brainly.com/question/24653699
molarity:
brainly.com/question/9324116
brainly.com/question/10608366
brainly.com/question/7429224
#SPJ1
what is the wavelength of light if the frequency is 7.0*10^16 Hz
Answers
49 becase you multilpy it
Explanation:
the neutralization reaction gets its name from.the fact that the products of the reaction are
Answers
Answer:
Neutral
Explanation:
Neutralization reaction gets its name from the fact that the products of the reaction are _____.
neutral
What is the balanced equation for the combustion of butane when the equation is balanced with the smallest, whole numbers possible
Answers
The balanced equation for the combustion of butane with the smallest whole numbers possible is:
2C4H10 + 13 O2 → 8 CO2 + 10 H2O.
Note that this equation is balanced because there are an equal number of atoms of each element on both sides of the equation.
The balanced equation for the combustion of butane with the smallest whole numbers possible is:
C4H10 + 13/2 O2 → 4 CO2 + 5 H2O.
However, since we need whole numbers, we can multiply the entire equation by 2 to achieve this:
2(C4H10) + 13(O2) → 8(CO2) + 10(H2O)
So, the final balanced equation with whole numbers is:
2C4H10 + 13O2 → 8CO2 + 10H2O
The equation shows that when two molecules of butane (C4H10) react with 13 molecules of oxygen (O2), they produce eight molecules of carbon dioxide (CO2) and 10 molecules of water (H2O).
The coefficients in front of each compound represent the number of molecules involved in the reaction.
To know more about balanced equation visit
https://brainly.com/question/28215760
#SPJ11
give the correct isotope symbol to identify and atom that contains 4 protons, 4 electrons, and 5 neutrons
Answers
The correct isotope symbol for the atom with 4 protons, 4 electrons, and 5 neutrons is 9Be.
The correct isotope symbol to identify an atom which contains 4 protons, 4 electrons, and 5 neutrons would be; 9Be
Here's a breakdown of the isotope symbol;
The atomic number is represented by the subscript, which indicates the number of protons. In this case, it is 4, so the symbol starts with the number 4.
The element symbol is Be, which represents the element beryllium.
The mass number will be the sum of the protons as well as neutrons. In this case, it is 4 protons + 5 neutrons = 9.
The mass number is usually written as a the superscript to left of the element symbol.
Therefore, the correct isotope symbol for the atom with 4 protons, 4 electrons, and 5 neutrons is 9Be.
To know more about isotope symbol here
https://brainly.com/question/29851218
#SPJ4
Which of the following statements is true about chemical nutrients in an ecosystem?
A. They cannot be obtained from decomposition.
B. They flow through the system, losing some nutrients in the process.
C. They exit the ecosystem in the form of heat.
D. They recycle within the ecosystem, being constantly reused.
E. They depend on sunlight as their source.
Answers
The statement that is true about chemical nutrients in an ecosystem is : D.) They recycle within the ecosystem, being constantly reused. Therefore, option D) is the correct answer.
The nutrient cycle is vital to the ecosystem, and this is how nutrients are recycled in it. Nutrients that are considered chemical nutrients include carbon, hydrogen, oxygen, nitrogen, and phosphorus.What are chemical nutrients in an ecosystem
Chemical nutrients refer to essential elements that are found in an ecosystem's physical and chemical environment. These elements are necessary for life because they are responsible for different functions such as cell structure, the production of enzymes, and the production of hormones.
In conclusion, chemical nutrients recycle within the ecosystem, being constantly reused. Nutrient recycling helps to maintain the ecosystem's sustainability. It helps to maintain the balance of life forms within the ecosystem.
To know more about ecosystem, refer
https://brainly.com/question/842527
#SPJ11
What type of reaction is it called after a & b mix together on the surface of an object?
Answers
When a and b are mixed on the surface of an object, a chemical reaction takes place called a Combination reaction.
What kind of reaction results when a and b combine on an object's surface?When two or more compounds (reactants) combine to form a single component, this is known as a direct combination reaction, also known as a synthesis reaction (product). These processes are described by equations of form X + Y → XY (A+B → AB). A combination reaction occurs when two or more components combine to form a single compound. In other terms, a combination reaction is a chemical reaction that occurs when two or more atoms or compounds react to create a single component. In a combination reaction, two reactants combine to create a single product. When a and b are mixed on the surface of an object, a chemical reaction takes place called a combination reaction since a new substance ab is prepared by the combination of a and b.To learn more about Combination reactions refer to:
https://brainly.com/question/12842366
#SPJ4
Draw a simple model of an atom to show the position of each
of the constituents of the atom
Answers
Answer:
Please see attachment
Explanation:
You can draw something like the attachment. The nucleus is in the middle with both proton and neutron. The electron circles around the nucleus

Determine physiological temperature, 98.6 F in degree C
Answers
Answer:
37
Explanation:
( 98.6 - 32 ) × 5(100c) ÷ 9(180f) = 37
How many grams of calcium chloride are needed to produce 15.0 g of potassium chloride?CaCl(aq) + K2CO3(aq)2 KCl(aq) + CaCO3(aq)

Answers
Answer
B. 11.2 grams
Explanation:
Molar mass of CaCl = 110.98 g/mol
Molar mass of KCl = 74.5513 g/mol
From the balanced chemical equation of the reaction given;
1 mole CaCl produced 2 moles KCl.
In grams;
(1 mol x 110.98 g/mol) CaCl produce (2 mol x 74.5513 g/mol) KCl, i.e
110.98 g CaCl produced 149.10236 g KCl,
Therefore, the grams of CaCl needed to produce 15.0 g KCl will be:
\(\text{Grams of CaCl n}eeded\text{ }=\frac{110.98\text{ g }\times15.0\text{ g}}{149.10236\text{ g}}=11.2\text{ grams}\)Therefore, 11.2 grams of calcium chloride are needed to produce 15.0 g of potassium chloride.
What is the percent of Nitrogen in NH3?
Answers
Answer:
82.244%
Explanation:
The mass percent is an important method which is used to calculate the concentration of a solution. The mass percent of nitrogen in NH₃ is 82.2%.
What is mass percentage?The mass percentage of a particular component in a compound can be defined as the ratio of the mass of the particular component to the total mass of the compound.
The equation which is used to calculate the mass percentage is:
Mass percentage = Mass of the component / Total mass of the compound × 100
or
The mass percentage is also calculated as:
% Mass = Mass fraction × 100
Here the mass of 'N' = 14.00 g
The molar mass of NH₃ = 17.031 g/mol
% Mass of 'N' = 14.00 / 17.031 = 0.82
0.82 × 100 = 82.2 %
Thus the mass percentage of nitrogen is 82.2 %.
To know more about mass percentage, visit;
https://brainly.com/question/27429978
#SPJ7
Four gases were combined in a gas cylinder with these partial pressures: 3.5 atm N2, 2.8 atm O2, 0.25 atm At, and 0.15 atm He
Answers
Answer:
Total pressure in container = 6.7 atm
Explanation:
Given:
N₂ = 3.5 atm
O₂ = 2.8 atm
At = 0.25 atm
He = 0.15 atm
Find:
Total pressure in container
Computation:
Total pressure in container = N₂ + O₂ + At + He
Total pressure in container = 3.5 + 2.8 + 0.25 + 0.15
Total pressure in container = 6.7 atm
NEED HELP FOR QUIZ!!
Both physical and chemical changes are associated with changes in energy. Compare the energy changes of the system and surroundings that are associated with exothermic changes and with endothermic changes. Answer in 3 to 5 sentences
Answers
Exothermic changes give out energy to their surroundings, causing an increase in heat endothermic changes, take in energy, so the opposite takes place.
is the product the same with the original material? why compare the materials before and after exposing it (soaked and exposed potato)
Answers
the answer is no
Explanation:
the soaked potato is already immersed in water and is likely to get spoilt few days before the exposed potato.because a liquid medium has already been used. while the exposed potato still possesses its same properties as before
Which formula is an empirical formula?
A) CH2OHCH2OH
B) H2C204
C) H2CO3
D) CH3COOH
Answers
An empirical formula represents the simplest ratio of atoms present in a compound. To determine the empirical formula, we need to simplify the given formulas to their simplest ratios.
Let's analyze the options:
A) CH2OHCH2OH: This formula can be simplified to C2H6O2. However, it is not in its simplest ratio, so it is not an empirical formula.
B) H2C204: This formula is already in its simplest ratio, so it is an empirical formula.
C) H2CO3: This formula is also already in its simplest ratio, so it is an empirical formula.
D) CH3COOH: This formula can be simplified to C2H4O2. However, it is not in its simplest ratio, so it is not an empirical formula.
Therefore, the empirical formulas among the given options are B) H2C204 and C) H2CO3.
To know more about empirical formula click this link -
brainly.com/question/32125056
#SPJ11
Zr + O ⇒
B + O ⇒
Zn + C ⇒
Co + I ⇒
Mg + Br ⇒
Answers
WHAT- WHAT GRADE R YOU IN 0,0
an arctic weather balloon is filled with 24.6l of helium gas inside a prep shed. the temperature inside the shed is 7 degrees celsius. the balloon is then taken outside, where the temperature is 7 degrees celsius. calculate the new volume of the balloon. you may assume the pressure on the balloon stays constant at exactly 1 atm. round your answer to 3 significant digits.
Answers
The balloon's new volume is 24.6L. An arctic weather balloon being inflated using 24.6 litres of helium gas in a prep shed. The shed is seven degrees Celsius inside.
When the balloon is hauled outside, it is seven degrees Celsius outside. Any three-dimensional solid's volume is equal to how much room it occupies. One of these solids can be a cube, a cuboid, a cone, a cylinder, or a sphere. Chemical compounds are composed of a large number of comparable molecules (or molecular entities), which are composed of atoms from various elements bonded together by chemical bonds. Because of this, a molecule composed of atoms from a single element is not a compound.
v1/t1 = v2/t2
24.6/7 = v2/7
v2 = 24.6L
v1 = v2
Learn more about helium gas here
https://brainly.com/question/26408362
#SPJ4
why do molecules change speed
Answers
Answer:
Molecules change speed based on temperature and state of matter. The warmer they are, the faster they move and vice versa. Solids are at a lower temperature than gases and liquids, which means the molecules are moving slower, and hold together better, also explaining why solids aren't malleable.
The particles gather kinetic energy and accelerate as the temperature rises.
What is kinetic energy?Kinetic energy is defined as an object is the energy it has since it is in motion. It is explained as the amount of effort required to move a mass-based body from rest to the indicated velocity. The body keeps its kinetic energy, which it acquired during acceleration, unless its speed changes.
The actual average speed of the particles is influenced by both their mass and temperature; at a given temperature, larger particles travel more slowly than lighter ones. Temperature and the physical state of the materials affect the speed of molecules. They move more quickly in warmer temperatures and vice versa. Since solids have lower temperatures than gases and liquids, their molecules move more slowly and adhere to one another more tightly, which also explains why solids aren't bendable.
Thus, the particles gather kinetic energy and accelerate as the temperature rises.
To learn more about kinetic energy, refer to the link below:
https://brainly.com/question/15764612
#SPJ2
which formulas represent compounds that are isomers of each other?

Answers
Answer:
Option D.
Explanation:
Isomerism is a phenomenon where by two or more compounds have the same molecular formula but different structural patterns. The compounds involved are called isomers.
Option A has two different compound with two different molecular formula. Hence they are not isomers.
Option B has two different compound with two different molecular formula. Hence they are not isomers
Option C can not be called isomers because Isomerism can not occur in compound having just 1 carbon atom.
Option D has two different compound with the same molecular formula as C3H8O and their structure are different. Hence they areisomers.