Answers
Answer:
Step 1 of 6
(a)
The mass of benzene is  , so calculate the moles of benzene as follows:

The mass of toluene is, so calculate the moles of toluene as follows:

Now, calculate the mole fraction as follows:


Therefore, the mole fraction of benzene and toluene is  and  respectively.
Step 2 of 6
(b)
The formula to calculate the partial pressure is as follows:

Here,  is the partial pressure of benzene,  is the vapour pressure of pure benzene and  is the mole fraction of benzene.
Vapour pressure of pure benzene at  is.
Substitute the values in the equation as follows:

Therefore, the partial pressure is  .
Step 3 of 6
(c)
Vapor pressure of the solution at 1 atm is  .
When the total pressure of the vapour pressure of the mixture is  at a temperature, then, the solution boils. It corresponds to the boiling point of the solution.
Calculate the total pressure of the solution at  as follows:

Since, the total pressure is less than the atmospheric pressure, the solution will not boil at  .
Calculate the total pressure of the solution at  as follows:

Since, the total pressure is greater than the atmospheric pressure, the solution will boil at  .
Therefore, the boiling point of the solution is  .
Step 4 of 6
(d)
Mole fraction of benzene at  is calculated as follows:

Mole fraction of toluene at  is calculated as follows:

Therefore, the mole fractions of benzene and toluene are  and  respectively.
Step 5 of 6
(e)
Vapor pressure of benzene at  is  .
Partial pressure of benzene is calculated as follows:

Vapor pressure of toluene at  is  .
Partial pressure of toluene is calculated as follows:

Step 6 of 6
Weight composition of the vapour that is in equilibrium with the solution is calculated as follows:

Weight composition of the vapour that is in equilibrium with the solution is calculated as follows:

Explanation:
mark me as brainliest
Mole fraction benzene = 0.0499 / 0.0798 = 0.5.
Mole fraction toluene = 0.0499 / 0.0798 = 0.5
Related Questions
12.5 mL of 0.280 M HNO3 and 5.0 mL of 0.920 M KOH are mixed. Is the resulting solution acidic, basic or neutral?
Answers
Answer:
The resulting solution is basic.
Explanation:
The reaction that takes place is:
HNO₃ + KOH → KNO₃ + H₂OFirst we calculate the added moles of HNO₃ and KOH:
HNO₃ ⇒ 12.5 mL * 0.280 M = 3.5 mmol HNO₃KOH ⇒ 5.0 mL * 0.920 M = 4.6 mmol KOHAs there are more KOH moles than HNO₃, the resulting solution is basic.
The resulting solution is basic.
• It is known that KOH is a base and HNO3 is an acid, so when they mix they undergo a neutralization reaction.
• The reaction between there will be,
HNO3 + KOH ⇔ KNO3 + H2O
Based on the given information,
• The volume of HNO3 is 12.5 ml and the molarity is 0.280 M, and the volume of KOH is 5 ml and the molarity is 0.920 M.
Now 1 mole of HNO3 completely reacts with 1 mole of KOH,
The millimoles of HNO3 is,
\(= Molarity * Volume (in ml)\\= 0.280 * 12.5\\= 3.5 mmol\)
The millimoles of KOH is,
\(= Molarity * Volume (in ml)\\= 0.920 * 5.0\\= 4.6 mmol\)
Now it can be seen that 3.5 millimoles of HNO3 completely reacts with 3.5 millimoles of KOH. Now we are left with 4.6-3.5 = 1.1 mmol of KOH.
Thus, KOH is in excess amount present in the solution, and as it is basic in nature, therefore, the resultant solution would be basic in nature.
To know more about:
https://brainly.com/question/12665051
Air will expand about the same amount as propane with the same change in temperature over ordinary temperature ranges.
Answers
Answer:
Yes this is true
Explanation:
Answer:
i have the same question for chemistry, do you have the answer?
Explanation:
1. A gas having the following composition is burnt under a boiler with 50% excess air.
Component Mole%
CH4 70
C3H8 15
CO 15
What is the composition of the stack gas?
Answers
The composition of the stack gas are :
\(CH_4\)= 0.8713
\(C_3H_8\) = 0.0202
CO = 0.107
What is a mole fraction?The ratio of the number of moles of one component of a solution or other mixture to the total number of moles representing all of the components.
Assuming 100 g of the stack gas. Calculate the mass of each species in this sample according to their percentages.
Mass of \(CH_4\) : 70% of 100 g = 70 g
Mass of \(C_3H_8\) : 15% of 100 g = 15 g
Mass of CO : 15% of 100 g = 15 g
Now calculate the number of moles of each species:
Number of moles of \(CH_4\) : \(\frac{70 g}{16.04 g/mol}\) = 4.3 mole
Number of moles of \(C_3H_8\): \(\frac{15 g}{144.1 g/mol}\) = 0.10 mole
Mass of CO : \(\frac{15 g}{28.01 g/mol}\) = 0.53 mole
Now to calculate the mole fraction of each we use the formula:
Mole fraction of \(CH_4\): \(\frac{4.3}{4.935}\) = 0.8713
Mole fraction of \(C_3H_8\) : \(\frac{0.10}{4.935}\) = 0.0202
Mole fraction of CO : \(\frac{0.53}{4.935}\) = 0.107
Hence, composition of the stack gas are:
\(CH_4\) = 0.8713
\(C_3H_8\) = 0.0202
CO = 0.107
Learn more about mole fraction here:
https://brainly.com/question/13135950
#SPJ1
Which forces can be classified as intramolecular?
London dispersion forces
van der Waals forces
hydrogen bonds
covalent bonds
Answers
Answer:
D) Covalent Bonds
Explanation:
Quizlet says so
Among the given options the intramolecular force is covalent bonding. Covalent bond is formed between two nonmetals by sharing of electrons. All other given forces are intermolecular.
What is covalent bonding ?A covalent bond is formed between two non-metals through sharing of valence electrons between atoms. For example carbon and oxygen are non metals forming the covalent compound CO.
The number of shared pair of electron depends on the valence of each atoms. For example in HCl, both atoms shares one electron to each other.
In a covalent compound, if the difference in electronegativity between two atoms is significant, the shared pair of electrons attracts towards the highly electronegative atom and resulting charge separation make them polar compounds.
Find more on covalent compounds:
brainly.com/question/21505413
#SPJ6
How many moles of O2 are needed to react with 7.00 moles of Al ?
Answers
As per the balanced reaction of Al and oxygen gas, 4 moles of Al needs 3 moles of oxygen gas. Hence, 7 moles of Al needs 5.2 moles of O₂.
What is aluminum oxide ?Aluminum metal is more reactive towards oxygen and it forms oxides on its surface. The balanced chemical equation of the reaction between Al and oxygen is written as follows:
\(\rm 4 Al + 3O_{2}\rightarrow2 Al_{2}O_{3}\)
As per this reaction, 4 moles of aluminum needs 3 moles of oxygen molecule. Then, the number of moles oxygen molecule required to react with 7 moles of Al is :
no.of moles of O₂ = (7×3)/4 = 5.2
Therefore, 5.2 moles of oxygen gas is required to completely react with 7 moles of aluminum metal.
Find more on aluminum oxide:
https://brainly.com/question/9496279
#SPJ1
A solid substance formed from a solution is a(n)
a. reactant
b. equation
C. compound
d. precipitate
Answers
A solid substance formed from a solution is a(n).
Answer:D. Precipitate
#CARRYONLEARNING #STUDYWELLA solid substance formed from a solution is a(n)
Choosing:a. reactant
b. equation
C. compound
d. precipitate
Answer:D. Precipitate
#READINGHELPSWITHLEARNING #CARRYONLEARNING #STUDYWELLHow many grams of hydrogen gas are produced from 2.50 mol of water?
__Ca(s) + __H2O(l) → __Ca(OH)2(aq) + __H2(g)
Answers
Answer:
2.52 g H₂
Explanation:
To find the mass of hydrogen gas, you need to (1) convert moles H₂O to moles H₂ (via mole-to-mole ratio from reaction coefficient) and then (2) convert moles H₂ to grams H₂ (via molar mass from periodic table values). When multiplying the given value by the mole-to-mole ratio, you need to use the coefficients of the balanced equation. An reaction is balanced once there is an equal amount of each element on both sides.
(Step 1)
The unbalanced equation:
Ca(s) + H₂O(l) ----> Ca(OH)₂(aq) + H₂(g)
Reactants: 1 calcium, 2 hydrogen, 1 oxygen
Products: 1 calcium, 4 hydrogen, 2 oxygen
The balanced equation:
1 Ca(s) + 2 H₂O(l) ----> 1 Ca(OH)₂(aq) + 1 H₂(g)
Reactants: 1 calcium, 4 hydrogen, 2 oxygen
Products: 1 calcium, 4 hydrogen, 2 oxygen
(Step 2)
Molar Mass (H₂): 2(1.008 g/mol)
Molar Mass (H₂): 2.016 g/mol
(Step 3)
2.50 moles H₂O 1 mole H₂ 2.016 g
--------------------------- x ------------------------- x ---------------------- = 2.52 g H₂
2 moles H₂O 1 mole
How many mL of 2.25M H2SO4 are needed to react completely with 69.9g BaO2

Answers
Answer:
4 millllllermeeters jb
The [H3O+] of a solution with pH = 8.7 is
Answers
The [H3O+] of a solution with pH = 8.7 is approximately 1.995 x 10^-9 M.
What is ph solution ?
The pH of a solution is a measure of its acidity or basicity. It is defined as the negative logarithm (base 10) of the concentration of hydronium ions, [H3O+], in the solution. Mathematically, pH is expressed as:
pH = -log[H3O+]
where [H3O+] is the concentration of hydronium ions in moles per liter (M) of the solution. pH values range from 0 to 14, where a pH of 7 is considered neutral. A pH value less than 7 indicates acidity, while a pH value greater than 7 indicates basicity or alkalinity. The pH scale is logarithmic, meaning that a change of one unit in pH represents a ten-fold change in the concentration of hydronium ions. For example, a solution with a pH of 3 has ten times more hydronium ions than a solution with a pH of 4.
The pH of a solution is defined as the negative logarithm (base 10) of the concentration of hydronium ions, [H3O+], in the solution. The mathematical expression for this relationship is:
pH = -log[H3O+]
We can rearrange this equation to solve for [H3O+]:
[H3O+] = 10^(-pH)
Substituting the given pH value of 8.7 into this equation, we get:
[H3O+] = 10^(-8.7)
Using a calculator or logarithmic tables, we find that:
[H3O+] = 1.995 x 10^(-9) M
Therefore, The [H3O+] of a solution with pH = 8.7 is approximately 1.995 x 10^-9 M.
To know more about ph visit:-
https://brainly.com/question/172153
#SPJ1
2.
Which mixture could be a useful buffer in a solution?
acetic acid (CH3CO2H) and hydrochloric acid (HCl)
sodium hydroxide (NaOH) and elemental sodium (Na)
ammonia (NH3) and ammonium chloride (NH4Cl)
acetic acid (CH3CO2H) and ammonia (NH3)
Pls answer quickly
Answers
Ammonia (\(NH_3\)) and ammonium chloride (\(NH_4Cl\)) mixture could be a useful buffer in a solution. Option C
A buffer is a solution that can resist changes in pH when small amounts of acid or base are added. It consists of a weak acid and its conjugate base or a weak base and its conjugate acid. The buffer system works by the principle of Le Chatelier's principle, where the equilibrium is shifted to counteract the changes caused by the addition of an acid or a base.
In option A, acetic acid (\(CH_3CO_2H\)) is a weak acid, but hydrochloric acid (HCl) is a strong acid. This combination does not form a buffer because HCl is completely dissociated in water and cannot provide a significant concentration of its conjugate base.
Option B consists of sodium hydroxide (NaOH), which is a strong base, and elemental sodium (Na), which is a metal. This combination does not form a buffer as there is no weak acid-base pair involved.
Option D contains acetic acid (\(CH_3CO_2H\)), a weak acid, and ammonia (\(NH_3\)), a weak base. Although they are weak acid and base, they do not form a buffer system together as they are both weak acids or bases and lack the required conjugate acid-base pair.
Option C, ammonia (\(NH_3\)), is a weak base, and ammonium chloride (\(NH_4Cl\)) is its conjugate acid. This combination can form a buffer system. When ammonia reacts with water, it forms ammonium ions (NH4+) and hydroxide ions (OH-).
The ammonium ions act as the weak acid, while the ammonia acts as the weak base. The addition of a small amount of acid will be counteracted by the ammonium ions, and the addition of a small amount of base will be counteracted by the ammonia, thus maintaining the pH of the solution relatively stable.
Therefore, option C, consisting of ammonia (\(NH_3\)) and ammonium chloride (\(NH_4Cl\)), is the suitable mixture that could be a useful buffer in a solution.
For more such question on buffer visit:
https://brainly.com/question/13076037
#SPJ8
Explain why the following picture illustrates the relationships between voltage, current and resistance. (hint: start with Ohm's Law)

Answers
Answer:
Explanation:
We can see that this picture can be used to show Ohm's Law graphically so it illustrates Ohm's Law.
According to Ohm's Law electric current is directly proportional to voltage and inversely proportional to resistance.
Mathematically, V ∝ I,
or V=IR,
where, V ⇒ voltage difference between two points,
I ⇒ current flowing through the resistance,
R ⇒ proportionality constant or resistance.
According to the picture voltage ( SI unit Volt ) is supporting the current ( SI unit Ampier ) to move out from the barrier while the resistor ( SI unit Ohm )is acting as a barrier to its way . As it is satisfying the Ohm's Law it illustrates the relationship between voltage, current, and resistance.
What is the wavelength (in nm) of the photon absorbed for a transition of an electron from initial=1 that results in the least energetic spectral line in the
ultraviolet series of the H atom? Round your answer to 4 significant figures.
Answers
121.523 nm is the wavelength (in nm) of the photon absorbed for a transition of an electron.
\(n_1=1 ,n_2=2\)
\(1/\lambda=1.09737 *10^7*1/1^2 *1/2^2=8230275m^-1\)
\(\lambda=1/8230275\)
1.215x10^-7 m=121.523 nm
since the dating between wave frequency and wavelength is inverse, gamma rays have extraordinarily tiny wavelengths that are best a small portion of the size of atoms, whereas different wavelengths can extend as a long way as the universe. No of the medium they are passing via, electromagnetic radiation's wavelengths are commonly expressed in phrases of the vacuum wavelength, but this isn't always said explicitly.
The wavelength of electromagnetic radiation influences how it behaves. speed of mild = wavelength x frequency power = Planck's consistent x frequency Wave wide variety = 1/wavelength in cm. The wavelengths of different elements of the electromagnetic spectrum are displayed collectively with a difficult approximation of the wavelength size.
To know more about wavelength visit : brainly.com/question/12924624
#SPJ9
How many atoms of Chlorine are in 1.00 mol of Chlorine gas?
6.022 x 10∧23
3.01 x 10∧23
6.022 x 10∧24
Answers
Answer:
6.02 × 10²³ atoms Cl₂
Explanation:
Avagadro's Number - 6.022 × 10²³ atoms, molecules, formula units, etc.
Step 1: Define
1.00 mol Cl₂ (g)
Step 2: Use Dimensional Analysis
\(1.00 \hspace{3} mol \hspace{3} Cl_2(\frac{6.02(10)^23 \hspace{3} atoms \hspace{3} Cl_2}{1 \hspace{3} mol \hspace{3} Cl_2} )\) = 6.02 × 10²³ atoms Cl₂
Which statement is true about a reversible reaction? (5 points)
The forward and reverse reactions stop.
The product is a salt and the reactant is an acid.
The products react to re-form the original reactants.
The forward reaction continues but reverse reaction stops.
Answers
Answer:
C: The products react to re-form the original reactants.
Explanation:
A reversible reaction is one where the conversion of reactants to products as well as the conversion of products to reactants happen at the same time.
What this means is that the products of the reaction could react to reform the initial reactants.
Thus, option C is the correct answer.
Answer:
C
Explanation:
The products react to re-form the original reactants.
Evan answer it................................
Answers
Answer: answer what..
Answer:
evan..
Explanation:
Hydrogen cyanide gas can be made by a two stepprocess. First, ammonia is reacted with oxygen gas to give nitrogen monoxide and water vapor. In the next step, nitrogen monoxide is reacted with methane (CH4) to give hydrogen cyanide gas. The by-products are water and hydrogen gas. A) Write the balanced equation for the two reactions.
B) When 24.2 g of ammonia and 25.1 g of methane are used, how many grams of hydrogen cyanide can be produced?
Answers
Answer:
38.34 g of HCN are produced
Explanation:
Our reactants for the first reaction are:
NH₃ and O₂
Products are: H₂O and NO
Our reactants for the second reaction are:
NO and CH₄
Producs are: H₂, H₂O and HCN
The reactions are:
4NH₃ and 5O₂ → 6H₂O + 4NO
2NO and 2CH₄ → 2HCN + H₂ + 2H₂O
In the first step 4 moles of ammonia can produce 4 moles of NO, so ratio is 1:1
24.2 g . 1mol / 17 g = 1.42 moles of ammonia
We have produced 1.42 moles of NO.
25.1 g . 1 mol / 16 g = 1.57 moles of methane.
Ratio is 2:2. So, for 1.57 moles of methane, we need 1.57 moles of NO.
Moles are the same. As there is no enough NO, this is the limiting reactant.
Ratio with product is also 2:2.
Our 1.42 moles of NO have produced 1.42 moles of HCN.
We convert moles to mass: 1.42 mol . 27 g/mol = 38.34 g
What is the molecular formula of each of the following
compounds?
(a) empirical formula CH₂, molar mass = 84 g/mol
(b) empirical formula NH₂Cl, molar mass = 51.5 g/mol
Answers
(a) the molecular formula of the compound is C₆H₁₂.
(b) the molecular formula of the compound is NH₂Cl.
(a) Given the empirical formula CH₂ and a molar mass of 84 g/mol, we need to determine the molecular formula. To do so, we need to find the factor by which the empirical formula needs to be multiplied to achieve the given molar mass.
The empirical formula CH₂ has a molar mass of 14 g/mol (12 g/mol for carbon + 2 g/mol for hydrogen).
To find the factor, we divide the molar mass by the empirical formula mass:
Factor = (molar mass) / (empirical formula mass) = 84 g/mol / 14 g/mol = 6
Therefore, the molecular formula is obtained by multiplying the empirical formula by the factor:
CH₂ × 6 = C₆H₁₂
Thus, the molecular formula of the compound is C₆H₁₂.
(b) Given the empirical formula NH₂Cl and a molar mass of 51.5 g/mol, we follow a similar approach.
The empirical formula NH₂Cl has a molar mass of 51.5 g/mol (14 g/mol for nitrogen + 2 g/mol for each hydrogen + 35.5 g/mol for chlorine).
To find the factor, we divide the molar mass by the empirical formula mass:
Factor = (molar mass) / (empirical formula mass) = 51.5 g/mol / 51.5 g/mol = 1
Therefore, the molecular formula is the same as the empirical formula: NH₂Cl
Hence, the molecular formula of the compound is NH₂Cl.
for more questions on molecular
https://brainly.com/question/24191825
#SPJ8
Which sample would you expect to have the smallest amount of kinetic energy?
THIS IS THE ANSWER. NOT A QUESTION
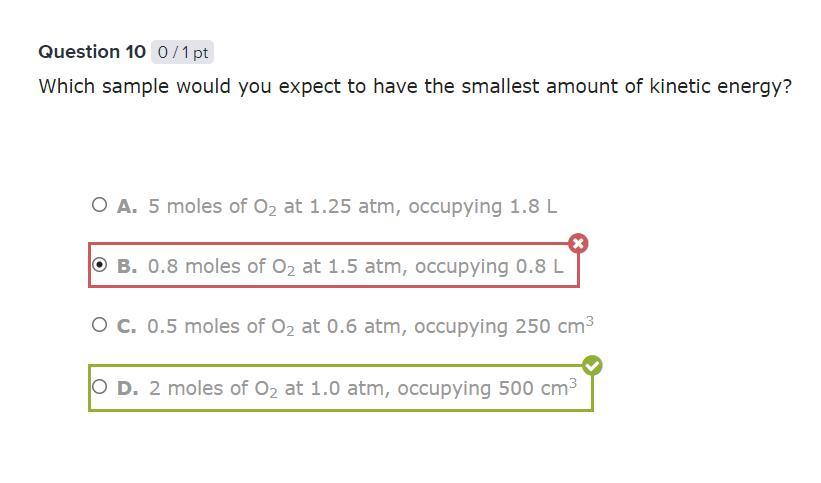
Answers
Answer:
ok so what do i do now that you posted the answer?
Answer: D
Explanation:
definitely didn't copy you
Pewter is a solidified solution of tin and lead or tin and zinc. In both cases, tin is the main component. Which metal would you classify as the solute in each type of pewter?
Answers
Which halogens are gases at STP?
Answers
As the atomic number increases, the reactivity of the halogens decreases. Fluorine and chlorine exist as gases at room temperature, while bromine is a liquid, and iodine is a solid.
Answer:
As the atomic number increases, the reactivity of the halogens decreases. Fluorine and chlorine exist as gases at room temperature, while bromine is a liquid, and iodine is a solid.
In the 1920s, western powers began to expand their influence in the middle east primarily due to __________. A. The discovery of oil in the regionb. The rising number of terrorist attacks against the westc. European desires to create a jewish homelandd. Rising fundamentalist movements in iran and saudi arabia please select the best answer from the choices provided. Abcd.
Answers
In the 1920s, western powers began to expand their influence in the middle east primarily due to A. The discovery of oil in the region.
The Role of Oil in the Expansion of Western Powers in the Middle East during the 1920sIn the 1920s, the Western powers, such as Britain and France, began to expand their influence in the Middle East primarily due to the discovery of oil in the region. The discovery of oil brought enormous economic and strategic importance to the region, and the Western powers sought to secure their access to this valuable resource. The discovery of oil also helped to modernize the region, as the Western powers invested in infrastructure development, such as pipelines, ports, and railways, which facilitated the transportation of oil and other goods. The Western powers also sought to protect their interests in the region by establishing protectorates, such as Kuwait and Bahrain, and by dividing up the former Ottoman Empire into new nation-states that would be more amenable to Western influence. This led to a backlash from some in the region, who saw the Western powers as meddling in their affairs, and who called for greater independence and self-determination. Nonetheless, the discovery of oil in the region had a profound impact on the political and economic development of the Middle East and continues to shape the region's geopolitics today.
To know more about the discovery of oil, visit:https://brainly.com/question/11429760
#SPJ4
Define biotechnology. } List two advantages in the use of biotechnology
Answers
Advantages of biotechnology:
Improvement of plants and animal breeds to give a high yield of their products.
Pests and pathogen control in agriculture which will reduce the loss of yield in food crops.
Synthesis of biocatalyst which can be used for enhancing the reactions which can be carried out in vitro or laboratory conditions.
Sewage treatment or water recycling can be done with the help of transgenic microbes which have better efficiency and speed.
Biotechnology is the use of living organisms or other biological systems in the manufacture of drugs or other products or for environmental management, as in waste recycling: includes the use of bioreactors in manufacturing, microorganisms to degrade oil slicks or organic waste, genetically engineered bacteria to produce human hormones, and monoclonal antibodies to identify antigens.
Biotech offers the possibility of improving human health, the environment, and agriculture while creating more sustainable modes of production.
Consider the following chemical equilibrium:
N2 (g) + 3H2 ⇌ 2NH3
Now write an equation below that shows how to calculate Kp from Kc for this reaction at an absolute temperature .
Answers
Answer:
Kp = Kc (RT) ^(-2)
Explanation:
For the reaction;
N2 (g) + 3H2 ⇄ 2NH3(g)
We can write;
Kc = [NH3]^2/[N2] [H2]^3
But
Kp = pNH3^2/pN2 . PH2^3
To convert from Kc to Kp
Kp = Kc (RT) ^Δn
where Δn is the change in number of moles going from reactants
to products.
For this reaction;
Δn = 2- (3+1) = -2
Kp = Kc (RT) ^(-2)
These type of cells lack a nucleus -
Answers
Answer:
Prokaryotes are cells that lacks nucleus
Explanation:
Hope you have a great amazing day and winter break:)
Describe another way you could collect or determine pressure data without using a gas pressure sensor, involving the same chemical reaction.
Answers
Another way you could collect or determine the pressure data without using a gas pressure sensor involving the same chemical reaction is using the Ideal Gas Equation.
A gas pressure sensor is a device used to monitor and collect data on the pressure changes and variations in a gas participating in a chemical reaction.
In a chemical reaction, the Ideal Gas Equation can be used to determine the pressure data without using a gas pressure sensor if the variables are known.
For example;
the numbers of moles(n) participating in the reaction, the volume(V), and;the temperature(T) at which the reaction is being carried outThe Ideal gas equation can be represented as:
PV = nRTwhere;
P = pressure data of the gasV = volume of the gasn = number of molesR = gas rate constantT = temperature of the gas.Therefore, we can conclude that the Ideal Gas Equation can be used to determine the pressure data without using a gas pressure sensor if the variables are known.
Learn more about the gas pressure sensor here:
https://brainly.com/question/18882857?referrer=searchResults
Convert 100.6 Kelvin to degrees C.
°C = K - 273
[?] °C
Answers
Answer:
-172.6 °C
Explanation:
You want to know the Celsius equivalent of the temperature 100.6 K.
ConversionThe relation is ...
C = K - 273.15
C = 100.6 -273.15 = -172.55
The temperature is -172.55 °C, about -172.6 °C.
__
Additional comment
We have rounded to tenths, because that is precision of the temperature given. If you use 273 as the conversion constant, you will get -172.4.
Draw structures for (a) a chain isomer, (b) a positional isomer, and (c) a functional isomer of hexan-1-ol
(i.e., 1-hexanol)
a. Chain isomer
b. Positional isomer
c. Functional isomer
Answers
Answer:
See attached picture.
Explanation:
Hello,
In this case, we should define each type of structural formula as shown below:
- Chain isomers: molecules with the same molecular formula, but different arrangements.
- Positional isomers are constitutional isomers that have the same carbon skeleton and the same functional groups but differ from each other in the location of the functional groups.
- Functional isomers are structural isomers that have the same molecular formula (that is, the same number of atoms of the same elements), but the atoms are connected in different ways so that the groupings are dissimilar.
Regards.

What physical property is characteristic of all of the elements in the group located in the rightmost column of the periodic table?
Answers
The physical property that is characteristic of all of the elements in the group located in the rightmost column of the periodic table is the gaseous state at room temperature.
What are Physical properties?Physical properties may be defined as those properties of matter that do not involves any chemical manifestation or appearance within the element. These properties are measurable and state the alteration between momentary states. Some examples of physical properties are as follows:
Physical appearance or color.Hardness of elementDensity.Melting and boiling points.Electrical conductivity.The groups located in the rightmost column of the periodic table are known as the halogens and the noble gases. These elements are typically gaseous in phase at room temperature. Apart from this, halogens also have high electronegativities because these elements have seven electrons in their valence electrons.
Therefore, the gaseous state at room temperature is the physical property that is characteristic of all of the elements in the group located in the rightmost column of the periodic table.
To learn more about the Modern periodic table, refer to the link:
https://brainly.com/question/15987580
#SPJ6
Please help (see file attached!!)

Answers
A = 2.86 x \(10^{23\) molecules of \(KMnO_4\)
B = 323.76 grams of Al
C = 1.53 moles of Au
D = 7.71 x \(10^{23\) molecules of \(H_3PO_4\)
E = 4.52 x \(10^{23\) total atoms
Avogadro's moleculesAccording to Avogadro, a single mole of any substance contains 6.022 x \(10^{23\) molecules or atoms of the substance.
Also, the mole of a substance is the ratio of the mass of the substance and the molar mass of the substance. In other words;
mole = mass/molar mass
These theories are what we need to solve the outlined problems.
Molar mass of \(KMnO_4\) = 158 g/molMole of 75 g of \(KMnO_4\):
mass/molar mass = 75/158
= 0.4747 mol
Since 1 mole = 6.022 x \(10^{23\) molecules
0.4747 mole = 0.4747 x 6.022 x \(10^{23\)
= 2.86 x \(10^{23\) molecules
7.23 x \(10^{24\) Al atomsSince 1 mole = 6.022 x \(10^{23\) molecules or atoms
7.23 x \(10^{24\) atoms = 7.23 x \(10^{24\) / 6.022 x \(10^{23\)
= 12 moles
Mass of 12 moles Al = 12 x 26.98
= 323.76 grams
9.23 x \(10^{23\) Au atoms = 9.23 x \(10^{23\)/6.022 x \(10^{23\)= 1.53 moles
125 g of \(H_3PO_4\) = 125/97.99= 1.28 moles
1.28 moles = 1.28 x 6.022 x \(10^{23\)
= 7.71 x \(10^{23\) molecules
0.75 moles of \(CO_2\) = 0.75 x 6.022 x \(10^{23\)= 4.52 x \(10^{23\) atoms
More on Avogadro's molecules can be found here: https://brainly.com/question/11907018
#SPJ1
13. When a electricity is passed through molten NaCl in the presence of CaCl₂ in the ratio of 2:3 by weight using graphite anode and iron cathode as electrodes, sodium metal is deposited at cathode and chlorine gas is liberated at anode in the electrolytic cell. a) Define electrolytic cell. (1) b) Find the mass of sodium metal deposited at cathode when 0.1 ampere of current is passed for half an hour and the process has 75% efficiency. (2) Why does calcium metal not deposit instead of sodium at cathode? d) Aqueous solution of sodium chloride can not be instead of molten sodium chloride for the same intended product ? Give reason. (1) (1)
Answers
An electrolytic cell is an electrochemical cell that forces a chemical reaction that wouldn't happen otherwise by using an external source of electrical energy.
What is the mass of sodium metal deposited at the cathode?The mass of sodium metal deposited at the cathode is calculated from the formula below:
mass of substance = (current * time * atomic mass) / (number of electrons * Faraday's constant * efficiency)
The mole of the electrons is obtained from the equation of the reaction as follows;
Na⁺(aq) + e⁻ → Na(s)The Moles ratio of electrons to sodium discharged is 1 : 1
Quantity of charge is 0.1 A × 30 min × 60 s/min = 180 C
One mole of electrons is equivalent to 1 Faraday constant:
1 mol e⁻ = 96,485 C
Therefore, the number of moles of electrons is:
180 C / 96,485 C/mol = 0.001866 mol e⁻
The molar mass of sodium is 22.99 g/mol.
So, the mass of sodium metal deposited at the cathode is:
Mass of metal deposited = (0.001866 mol × 22.99 g/mol) / (1 × 96,485 C/mol × 0.75)
Mass of metal deposited = 0.000365 g
Calcium metal does not deposit instead of sodium at the cathode because sodium metal is more electropositive than calcium.
An aqueous solution of sodium chloride cannot be used instead of molten sodium chloride to obtain the same products because sodium is not discharged in preference of hydrogen ions.
Learn more about electrolytic cells at: https://brainly.com/question/21722989
#SPJ1