What is the molarity of the solution made by dissolving 15.1 g of solid naf in water and diluting it to a final
volume of 550.0 ml?
Answers
The molarity of the solution is 0.5 M.
To calculate the molarity of the solution, we need to first calculate the number of moles of NaF present in the solution. The molar mass of NaF is 41.99 g/mol (22.99 g/mol for Na and 19.00 g/mol for F).
Number of moles of NaF = mass of NaF / molar mass of NaF
= 15.1 g / 41.99 g/mol
= 0.359 mol
The volume of the solution is given as 550.0 mL, which needs to be converted to liters (L) as the unit of molarity is moles/L.
Volume of the solution = 550.0 mL = 0.5500 L
Molarity of the solution = number of moles of solute / volume of solution
= 0.359 mol / 0.5500 L
= 0.653 M
However, we need to consider that the NaF was diluted to a final volume of 550.0 mL, which means that the concentration of the solution has been decreased. Therefore, we need to divide the calculated molarity by 2.
Molarity of the solution after dilution = 0.653 M / 2
= 0.5 M
To know more about molarity, refer here:
https://brainly.com/question/30404105#
#SPJ11
Related Questions
Be sure to answer all parts.
Determine the partial pressure and number of moles of each gas in a 14.75−L vessel at 30.0°C containing a mixture of xenon and neon gases only. The total pressure in the vessel is 4.70 atm, and the mole fraction of xenon is 0.701.
What is the partial pressure of xenon?
atm
What is the number of moles of xenon?
mol
What is the partial pressure of neon?
atm
What is the number of moles of neon?
mol
Answers
The partial pressure of xenon is 3.29 atm.
The number of moles of xenon is 5.45 mol.
The partial pressure of neon is 1.41 atm.
The number of moles of neon is 9.24 mol.
Using Dalton's law of partial pressures, the total pressure is the sum of the partial pressures of each gas. Let P_Xe and P_Ne be the partial pressures of xenon and neon, respectively. Then we have:
P_Xe + P_Ne = 4.70 atmThe mole fraction of xenon is given as 0.701, which means that the mole fraction of neon is 0.299. Therefore, we can write:
Xe moles / Total moles = 0.701Ne moles / Total moles = 0.299We can solve for the number of moles of each gas:
Xe moles = 0.701 × Total molesNe moles = 0.299 × Total molesWe can substitute these expressions into the equation for partial pressures:
P_Xe = Xe moles / Total moles × Total pressureP_Ne = Ne moles / Total moles × Total pressurePlugging in the given values, we get:
P_Xe = 0.701 × 4.70 atm = 3.29 atmXe moles = 0.701 × 14.75 L / 0.08206 L·atm/mol·K × (30.0°C + 273.15) K = 5.45 molP_Ne = 0.299 × 4.70 atm = 1.41 atmNe moles = 0.299 × 14.75 L / 0.08206 L·atm/mol·K × (30.0°C + 273.15) K = 9.24 molTo learn more about partial pressure, here
https://brainly.com/question/31214700
#SPJ1
Organisms change energy from the environment or from their food into other types of energy. This is called what?
Answers
Answer:
Metabolism
Explanation:
This is called Metabolism.
Metabolism is a combination of spontaneous chemical reactions which release non-spontaneous energy and chemical reactions requiring energy to proceed. In order to perform cellular processes, living organisms can take energy from food, nutrients or sunlight.
An investigation into an unknown object in space resulted in the following observations:
It is made of rock.
It has a round shape.
What additional piece of information is required to confirm that the object is an asteroid?
It gives off light.
It has a tail of dust and gas.
It orbits the sun.
It rotates on its axis.
Answers
An investigation into an unknown object in space resulted in the following observations: It was discovered to be an asteroid, It revolves around the sun, which is in the third position.
What is an asteroid?It is a small, rocky substance that rotates around the sun and is found in the asteroid belt, which is located between the orbits of Mars and Jupiter, and its size varies, with some still in fragments and others having a large structure.
Hence, an investigation into an unknown object in space resulted in the following observations: It was discovered to be an asteroid, It revolves around the sun, which is in the third position.
Learn more about the asteroid here.
https://brainly.com/question/19161842
#SPJ1
1. what would happen to an enzyme if the temperature and Ph changed significantly beyond the enzyme’s optimum level?
2. How would this affect enzyme activity?
Answers
i am groot i am groot
The use of phenol (carbolic acid) as a surgical wound disinfectant was first practiced by?
Answers
The use of phenol (carbolic acid) as a surgical wound disinfectant was first practiced by Joseph Lister.
Who was Joseph Lister?
Joseph Lister was a surgeon, scientist, pathologist, etc. He was famous for the discovery of the prevention of infection in wounds. He first used phenol as a disinfectant agent for wounds.
Phenol is an inorganic compound, it is white crystalline and solid. It was used by Joseph Lister as an agent for a disinfectant agent. He discovered the safer medical procedure.
Thus, Joseph Lister was the first to utilize phenol (carbolic acid) to sterilize surgical wounds.
To learn more about Joseph Lister, refer to the below link:
https://brainly.com/question/472334
#SPJ4
The whole reason things stay afloat or sink is because water is
Answers
Answer:
I think the density of the object determines if it floats or sinks
The amount of water flowing through a channel over a given amount of time is called its ______.
Answers
Answer:
Discharge
Explanation:
this is the answer
If the sun produces energy by the proton-proton chain, then the center of the sun must have a temperature of at least
a.
104 K
b.
107 K
c.
1010 K
d.
1013 K
e.
1016 K
Answers
b. 10^7 K
Explanation:
The proton-proton chain is the primary nuclear reaction that occurs in the sun, which converts hydrogen into helium and produces energy in the process. The temperature required to sustain this reaction is very high.
According to current scientific understanding, the temperature at the core of the sun is approximately 15 million degrees Celsius (or about 27 million degrees Fahrenheit). This corresponds to a temperature of about 10^7 K, which is option (b).
Therefore, the correct answer is (b) 10^7 K.
what is the mass of 1 mole of water
Answers
Answer:
18.01528 g/mol
Explanation:
Using the equation to figure this out
Answer:
18.01528 g/mol
In which type of chemical reaction do the positive ions switch between two compounds and form two brand new compounds?
Answers
In double displacement type of reaction the positive ions switch between two compounds and form two brand new compounds.
A double displacement is defined as a reaction in which the positive and negative ions of two ionic compounds exchange places to form two new compounds.
The double-displacement reaction generally represented in the form of AB + CD → AD + CB where A and C are positively-charged cations, while B and D are negatively-charged anions.
Some of the examples of double displacement reaction are given as,
NaOH + NH₄Cl ⇄ NaCl + NH₄OH
AgNO₃ + NaCl ⇄ AgCl + NaNO₃
Learn more about double displacement reaction from the link given below.
https://brainly.com/question/14195530
#SPJ4
Someone help me quick! Will give brainliest
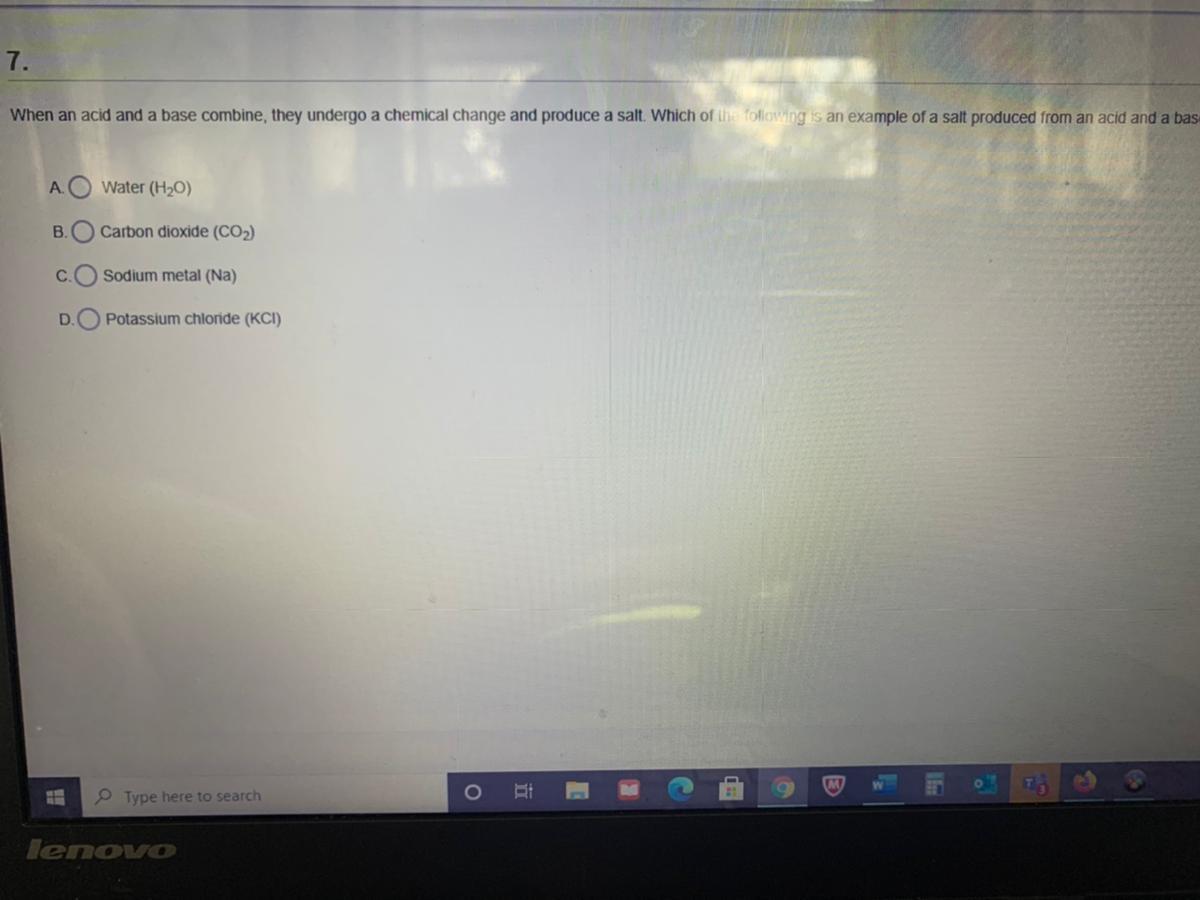
Answers
Answer:
WHY DO YOU NEED HELP HUH
DON'T CHEAT READ AND STUDY
Explanation:
Answer:
It's C
Explanation:
Will autoionization of water be spontaneous at high temperatures
Answers
Answer:
100
Explanation:
Will autoionzation of water be spontaneous at high temperature
Write the advantages of modern periodic table write as more as you can and don't copy the same thing
Answers
Answer:
the advantages of modern periodic tables are given below and explained.
position of hydrogen:since hydrogen has the least atomic number i.e 1 ,it is kept in group 1 of modern periodic table, but still controversial due to its. dual characteristics since it shows the characteristics of borh group 1 and group 17.position of isotopes :isotopes are element having the same atomic number but different atomic weight . Without any doubt all isotopes of one element and kept in one place.position of lanthanide and acnitinides: element of Lanthanides series and element of Actinides series are kept below the main block of the periodic table as they have different properties from other elements.correction of periodic law: some elements do not obey mendeleev periodic law , but when they are arranged according to atomic number they obey the modern periodic law.position of alkali metals and coinage metals : in modern periodic table , alkali metal are kept in group IA and coinage metals are in group.hope this helped you☺️
any confusion then comment it and let me know.
actually as I say these points say that the modern periodic table is better than mendeleev periodic table so, don't get confused.
Describe three ways in which colorless compounds can be located ona TLC slide:
Answers
Three ways in which colorless compounds can be located on a TLC (Thin Layer Chromatography) slide are:
1) Iodine Staining: The TLC slide is placed in a container with iodine crystals. The iodine vapor reacts with the colorless compounds and produces a yellowish-brown stain, which helps to locate the compounds.
2) UV Light: The TLC slide is exposed to UV light. Some colorless compounds absorb the UV light and appear as dark spots on the slide.
3) Chemical Staining: The TLC slide is treated with a chemical reagent that reacts with the colorless compounds and produces a colored stain. For example, ninhydrin is used to locate amino acids and proteins on a TLC slide.
In conclusion, colorless compounds can be located on a TLC slide by using iodine staining, UV light, and chemical staining. These methods help to visualize and identify the compounds on the slide.
To know more about TLC click here:
https://brainly.com/question/13086570
#SPJ11
What is the correct name for LiF?
Answers
Answer:
Lithium Fluoride.
Explanation:I know science.
Select the single best answer.
Predict the pH (> 7, < 7, or = 7) of a LiHCO3 solution.
Answers
Answer:
LiHCO3 its PH >7
Explanation:
the real ph for it is 11.3 so it makes it more than 7
and if you know it is a conjugate base coming from the weak acid H2CO3 so LiHCO3 is a base
How would you describe light generated by heating pure elements if it was observed through a prism or spectroscope?
Answers
If you were to observe light generated by heating pure elements through a prism or spectroscope, you would notice a unique spectral pattern. The spectral pattern would appear as a series of colored lines separated by dark spaces, and this is known as the atomic spectrum of the element.
Each pure element has its own distinct atomic spectrum, which arises due to the arrangement of electrons in the element's atoms. The electrons in the atoms occupy energy levels, and when they transition between these levels, they emit or absorb light at specific wavelengths. These wavelengths correspond to the different colors observed in the atomic spectrum. Therefore, the use of a prism or spectroscope can reveal valuable information about the composition of the element, as well as its electronic structure. Overall, studying the spectral patterns of different pure elements can provide insight into the fundamental building blocks of matter and the interactions of atoms with light.
To know more about Elements visit:
https://brainly.com/question/8460633
#SPJ11
Rachel was riding her bike at a velocity of 9 m/s to the east. Her velocity
changed to 6 m/s to the east. What was her change in velocity?
A. 9 m/s east
B. 6 m/s east
C. -3 m/s east
D. 3 m/s east
Answers
Answer:-3m/s east
Explanation:that’s right
Explain the effect of carbon dioxide on the ph of the oceans.
Answers
Answer:
When carbon dioxide dissolves in seawater, the water becomes more acidic and the ocean's pH (a measure of how acidic or basic the ocean is) drops.
Explanation:
hope this helps
How many molecules of carbon dioxide gas, CO2, are found in 0.125 moles
Answers
There are 7.52 x 10^22 molecules of carbon dioxide gas, CO2, in 0.125 moles.
The number of molecules in a given number of moles can be calculated using Avogadro’s number, which is approximately 6.022 x 10^23. This number represents the number of particles (atoms or molecules) in one mole of a substance.
To calculate the number of molecules in 0.125 moles of CO2, we can multiply the number of moles by Avogadro’s number: 0.125 moles x (6.022 x 10^23 molecules/mole) = 7.52 x 10^22 molecules.
Avogadro’s number is a fundamental constant in chemistry and is used in many calculations involving moles and molar mass.
To learn more about carbon dioxide,
brainly.com/question/3049557
In the graphic X represents which element? 31 15 11x А B 31 Gallium 15 Phosphorus P 46 Palladium Pd Ga

Answers
Answer:
B. X represents phosphorus
Explanation:
In an isotope symbol, you write the mass number above the atomic number. So the 31 represents the mass of the protons and neutrons of the element added together, and the 15 represents the atomic number or the total number of protons in the nucleus.
For each of these pure substances, list the atoms that are in one molecule
of the substance. Say whether it is an element or a compound.
e) Na
a) H₂O
b)CO₂
c) H₂
d) C₂H₁2O6
Answers
A)compound
B) compound
C) element
D)compound
The Calories on a food label indicate the
amount of energy the food contains in
one serving. Which form of energy is
contained in food?
Answers
Answer:
chemical energy
Explanation:
if 28.0% of a sample of silver-112 decays in 1.52 hours, what is the half-life of this isotope (in hours)?
Answers
By dividing the amount of half-lives by the time it took for two half-lives to pass (3.04 hours), we can determine the half-life of silver-112. (2). This gives us a silver-112 half-life of 1.52 hours.
The half-life of a radioactive isotope is defined as the amount of time it takes for half of the initial amount of the isotope to decay. To determine the half-life of silver-112, we can use the information given in the problem.
We know that 28.0% of a sample of silver-112 decays in 1.52 hours. Let's assume that we started with a sample of 100 silver-112 atoms. This means that 28.0 of these atoms would decay in 1.52 hours, leaving us with 72.0 atoms remaining.
After another half-life, we would expect half of the remaining atoms to decay. In other words, we would expect 36.0 atoms to decay, leaving us with 36.0 atoms remaining. Since we started with 100 atoms and now have 36.0 remaining, this means that two half-lives have passed.
Therefore, we can calculate the half-life of silver-112 by dividing the time it took for two half-lives to pass (3.04 hours) by the number of half-lives (2). This gives us a half-life of 1.52 hours for silver-112.
To learn more about half-life refer to:
brainly.com/question/24710827
#SPJ4
this imagine shows the continents breaking apart what do scientist think causes the continents to move?

Answers
The movement of the continental plates is due to convection currents located at the Earth's mantle below the crust.
A tectonic plate is a slab of solid rock consisting of continental and oceanic lithosphere. The continental and oceanic plates include, among others, the Pacific plate, Nazca plate, the Eurasian plate, the Australian-Indian plate, the North American plate, the South American plate, the African plate, the Antarctic plate, etc.The convection currents control the movement of the continental and oceanic plates on the Earth's molten mantle.In conclusion, the movement of the continental plates is due to convection currents located at the Earth's mantle below the crust.
Learn more about the continental plates here:
https://brainly.com/question/10508547
what type of reaction is 2 C5H5 + Fe --> Fe(C5H5)2
Answers
Answer:
Combination or Synthesis
Explanation:
It is a combination because 2 reactants are combining together to form the product.
A hot metal is placed in 100.0 g sample of water and it causes the temperature of the water to increase from 25 C to 27 C. How many joules of energy did the water absorb from the metal?
Answers
Answer: \(836.8\ J\)
Explanation:
Given
Mass of water in the sample is \(m=100\ g\)
Temperature changes from \(25^{\circ}C\) to \(27^{\circ}C\)
Change in temperature is \(\Delta T=27-25=2^{\circ}C\ \text{or}\ 2\ K\)
We know, specific heat of water is \(c=4.184\ J/g-K\)
Heat absorbed is given by
\(\Rightarrow Q=mc\Delta T\\\Rightarrow Q=100\times 4.184\times 2\\\Rightarrow Q=836.8\ J\)
Thus, water absorbs \(836.8\ J\) of heat from hot plate.
answer the question in the picture

Answers
Since potassium bromide is replaced by element X to generate KX and Br2, we can infer from the reaction that element X is more reactive than bromine.
What is the right way to pronounce potassium bromide?As a result, potassium bromide is represented by the formula KBr, but when the potassium and bromide ions are written separately, they are K+ and Br.
Why does adding chlorine to an aqueous potassium bromide solution cause it to turn reddish brown?As a result, when chlorofluorocarbon gas is passed through a potassium bromide aqueous solution, bromine gas is released, which causes the solution to turn brown.
To know more about reaction visit:-
https://brainly.com/question/28984750
#SPJ1
give an example of a chemical change that you encounter every day
Answers
One example of a chemical change that we encounter every day is the process of combustion.
Combustion is a chemical reaction in which a substance reacts with oxygen to produce heat, light, and new chemical compounds. A common example is the burning of wood or paper.
When we light a match or ignite a piece of paper, the chemical bonds in the material break, and the carbon and hydrogen atoms in the substance combine with oxygen from the air to form carbon dioxide and water vapor.
This release of energy in the form of heat and light is a result of the chemical change that occurs during combustion, and it is something we encounter regularly in activities such as lighting a candle or cooking food on a gas stove.
To know more about combustion, refer here:
https://brainly.com/question/31123826#
#SPJ11
Express the dosage using the ratio format you prefer. (Use mg for milligrams and mL for an injectable solution that contains 250mg in each 0.6 mL 3. [-/3 Points] CURRENMEDMATH11 12.3.002. EP. Consider the following. A 40mg in 2.5 mL solution will be used to prepare a 26mg dosage. Calculate the dosage using ratio and proportion. Express your final answer in mL to the
40mg
mL
=
X mL
26mg
40x
X
=
=
mL
[-/1 Points] CURRENMEDMATH11 12.3.004. Calculate the dosage (in milliliters). Express your answer to the nearest tenth. Assess y A 36mg per 2 mL strength solution is used to prepare 22mg. mL
Answers
The dosage of 26mg can be prepared using approximately 1.625 mL of the 40mg in 2.5 mL solution.
The dosage of 22mg can be prepared using approximately 1.222 mL of the 36mg per 2 mL strength solution.
To calculate the dosage using ratio and proportion, we can set up a proportion based on the strength of the solution.
40mg in 2.5 mL solution will be used to prepare a 26mg dosage.
Let X represent the mL of the solution needed to prepare the 26mg dosage.
We can set up the proportion as follows:
40mg/2.5mL = 26mg/X mL
Cross-multiplying and solving for X, we have:
40mg * X mL = 2.5mL * 26mg
40X = 65
X = 65/40
X ≈ 1.625 mL
For the second question:
36mg per 2 mL strength solution is used to prepare 22mg.
Let Y represent the mL of the solution needed to prepare the 22mg dosage.
We can set up the proportion as follows:
36mg/2mL = 22mg/Y mL
Cross-multiplying and solving for Y, we have:
36mg * Y mL = 2mL * 22mg
36Y = 44
Y = 44/36
Y ≈ 1.222 mL
To know more about solution
https://brainly.com/question/1616939
#SPJ11