what is the maximum number of electrons that can occupy a principal of energy level (n) of an atom
Answers
Related Questions
Which of the following is a result of the specific heat differences between land and ocean?
A. Ocean tides are created.
B. Volcanoes are created.
C. Saltwater is created.
D. Breezes are created.
Answers
please help with stoichiometry!

Answers
Answer:
I don't know sorry
Explanation:
ask tutor
Which of the following reasons best explains why water isn
waxed surfaces? *
O the wax is a nonpolar substance that will not mix with polar wa
O the wax is a polar substance that will not mix with polar water
O the wax is a polar substance that will not mix with nonpolar wa
O the wax is a nonpolar substance that will not mix with nonpola
Answers
Answer:
the wax is a nonpolar substance that will not mix with polar water
Explanation:
Water is polar by due to the uneven distribution of charge between the hydrogen and oxygen. T=This automatically eliminates options 3 and 4.
Wax on the other hand is a non polar substance. Due to this non polar characteristic, it would not dissolve in water. The correct option is;
- the wax is a nonpolar substance that will not mix with polar water
What can you conclude about the ad disease from the cd4-deficient (cd4-ko) and cd8-deficient (cd-ko) mice?
Answers
CD4 and CD8 T-cell numbers are decreased after blunt trauma.
Blunt traumaA body damage brought on by a strong collision with a dull item or surface is known as blunt trauma, also known as non-penetrating trauma or blunt force trauma. It differs from penetrating trauma, which occurs when an object or surface pierces the body and leaves an open wound. To stabilize and care for the patient, trauma hospitals need to use a coordinated, multidisciplinary approach. For instance, a Level I trauma center has well-established treatment protocols in place and specific sorts of healthcare specialists on call around-the-clock, seven days a week.
Learn more about blunt trauma here:
https://brainly.com/question/17986674
#SPJ4
(1) what is the element with an electron configuration of 1s22s22p63s23p64s23d1? fill in the blank 1 (2) what is the element with an electron configuration of 1s22s22p3?
Answers
The first element whose atomic number is 21 is Scandium and the second element whose atomic number is 7 is Nitrogen. Based on electronic configuration
Electronic configuration is a systematic way of distributing the electrons in their orbitals Electron configurations of atoms follow a standard notation in which all electron-containing atomic subshells (with the number of electrons they hold written in superscript) are placed in a sequence.
Electron Configurations are useful for:
Determining the valency of an element.
Predicting the properties of a group of elements (elements with similar electron configurations tend to exhibit similar properties).
Interpreting atomic spectra.
To know about electronic configuration
https://brainly.com/question/21977349
#SPJ4
Monday
m
Use the green plane to answer questions 1-3
E
B
F
ח
С
1) What are 2 ways you can name the plane?
a) be
b) BE
2) How dle can you name line FE? (Bonus: Can you think of another way?)
EF
3) Name 3 points on the plane.

Answers
2)they are alike just different placement
3)it don’t continue because it makes a cube
What is the solution to the problem expressed to the correct number of significant figures?2.13 + 1 = ?A.3B.3.0C.3.1D.3.13
Answers
The correct answer to the problem expressed to the correct number of significant figures is option C, 3.1.
Significant figures are a way of measuring the precision of a value and indicate the digits that give meaning to a number. The rules for determining the number of significant figures are as follows:
1. All non-zero digits are significant.
2. Zeroes between non-zero digits are significant.
3. Trailing zeroes are significant if there is a decimal point.
4. Trailing zeroes are not significant if there is no decimal point.
5. In scientific notation, all digits are significant.
In the given problem, we have the addition of 2.13 and 1. The number 2.13 has two significant figures, and the number 1 has one significant figure. According to the rules, the result of the addition should have the same number of significant figures as the number with the fewest significant figures.
Performing the addition gives us 3.13. However, since the number 1 has only one significant figure, the result should be rounded to one significant figure after the decimal point. Therefore, the correct answer is 3.1.
The problem expressed to the correct number of significant figures yields the answer 3.1, rounded to one significant figure after the decimal point. This aligns with the rules for significant figures, which indicate that the result should have the same number of significant figures as the number with the fewest significant figures.
To know more about significant figures click here:
https://brainly.com/question/23396760
#SPJ11
prepare (your own) problem with its correct answer about (chromatography). must continue (calculations) please create a problem by yourself and solve it correctly //Don't copy paste from any sources cause that will not be accepted
Answers
In a chromatography experiment, a mixture of red, blue, and green dyes is separated using a stationary phase and a mobile phase. The stationary phase has a length of 10 cm, and the mobile phase moves at a constant velocity of 2 cm/min. The red dye travels a distance of 6 cm, the blue dye travels a distance of 8 cm, and the green dye travels a distance of 9.5 cm.
What is the retention factor (Rf) for each dye?
Solution:
To calculate the retention factor (Rf) for each dye, we use the formula:
Rf = Distance traveled by the dye / Distance traveled by the mobile phase
For the red dye:
Distance traveled by the dye = 6 cm
Distance traveled by the mobile phase = 10 cm
Rf (red) = 6 cm / 10 cm = 0.6
For the blue dye:
Distance traveled by the dye = 8 cm
Distance traveled by the mobile phase = 10 cm
Rf (blue) = 8 cm / 10 cm = 0.8
For the green dye:
Distance traveled by the dye = 9.5 cm
Distance traveled by the mobile phase = 10 cm
Rf (green) = 9.5 cm / 10 cm = 0.95
Therefore, the retention factors (Rf) for the red, blue, and green dyes are 0.6, 0.8, and 0.95, respectively.
You can learn more about chromatography experiment at
https://brainly.com/question/27007683
#SPJ11
Polar molecules have _____.
Select one:
a. very unstable structures
b. no charge
c. linear molecular structures
d. charges ( a slight positive charge on one end, and a slight negative charge on the other)
Answers
Answer:
B. Charges ( a slight positive charge on one end, and a slight negative charge on the other).
A metal crystallizes in the face‑centered cubic (FCC) lattice. The density of the metal is 12020 kg/m3, and the length of a unit cell edge, , is 389.08 pm. Calculate the mass of one metal atom in grams.
Answers
Answer:
1.7700×10^-22 g
Explanation:
A face-centered-cubic cell contains 4 atoms, so the volume per atom is ...
(389.80×10^-12 m)³/(4 atoms) ≈ 1.4725×10^-29 m³
Then the mass of 1 atom is ...
(12020 kg/m³)(1.4725×10^-29 m³) ≈ 1.76995×10^-25 kg
≈ 1.7700×10^-22 g
_____
Additional comment
That is the approximate mass of a Palladium atom.
Answer:
1.7700×10^-22 g
Explanation:
blank a long narrow Stepside value that forms the deepest part of the ocean
Answers
Answer:
The answer is a trench
Which sample of matter is a single substance
A air
B ammonia gas
C hydrochloric acid
D Salt water
Answers
Answer:
B) Ammonia gas
Explanation:
Ammonia gas: It consists of only one molecule namely ammonia throughout the substance so it is a pure single substance.
7. Which liquid caused the egg to shrink? *
(1 Point)
a. Vinegar
b.Water
c.Corn Syrup
Answers
Answer:
c.corn syrup
the egg shrinks due to osmosi.
A potassium-40 sample starts with 50 atoms, and 1.25 billion years later, there are 25 atoms. What is the half-life of potassium-40?
A. 2.5 billion years
B. 25 years
C. 40 years
D. 1.25 billion years
Answers
the temperature at which a liquid becomes a gas is called the
Answers
What are examples of the solar system
Answers
Answer:(:
Explanation:
An example of the solar system is the eight planets including Earth that revolve around the sun. The sun together with the eight planets and all other celestial bodies that orbit the sun. A system of planets or other bodies orbiting a star. That portion of our galaxy which is subject to the gravity of the sun
issued this? watch kcv: atomic theory; read section 2.3. you can click on the review link to access the section in your etext. carbon and oxygen form both carbon monoxide and carbon dioxide. when samples of these are decomposed, the carbon monoxide produces 3.36 g of oxygen and 2.52 g of carbon, while the carbon dioxide produces 9.92 g of oxygen and 3.72 g of carbon.
Answers
The atomic ratio of carbon to oxygen in carbon monoxide (CO) is 1:1, and the atomic ratio of carbon to oxygen in carbon dioxide (CO₂) is 2:1.
Firstly, we can analyze the decomposition of carbon monoxide (CO) and carbon dioxide (CO₂) to determine the atomic ratios involved.
Let's denote the atomic ratio of carbon to oxygen in carbon monoxide as x, and the atomic ratio of carbon to oxygen in carbon dioxide as y.
According to the given data;
Decomposition of carbon monoxide (CO);
Oxygen produced = 3.36 g
Carbon produced = 2.52 g
We know that the atomic mass of carbon is 12 g/mol, and the atomic mass of oxygen is 16 g/mol. Using these values, we can calculate the number of moles for each element;
Number of moles of oxygen = mass / atomic mass = 3.36 g / 16 g/mol = 0.21 mol
Number of moles of carbon = mass / atomic mass = 2.52 g / 12 g/mol = 0.21 mol
Since the atomic ratio of carbon to oxygen in carbon monoxide is x, we can write the following equation;
0.21 mol C / (0.21 mol O) = x
Simplifying the equation, we have;
x = 1
Therefore, the atomic ratio of carbon to oxygen in carbon monoxide is 1:1.
Decomposition of carbon dioxide (CO₂);
Oxygen produced = 9.92 g
Carbon produced = 3.72 g
Following the same calculations as before;
Number of moles of oxygen = mass / atomic mass = 9.92 g / 16 g/mol = 0.62 mol
Number of moles of carbon = mass / atomic mass = 3.72 g / 12 g/mol = 0.31 mol
Since the atomic ratio of carbon to oxygen in carbon dioxide is y, we can write the following equation;
0.31 mol C / (0.62 mol O) = y
Simplifying the equation, we have;
y = 0.5
Therefore, the atomic ratio of carbon to oxygen in carbon dioxide is 1:0.5, which can be simplified to 2:1.
To know more about decomposition here
https://brainly.com/question/20418092
#SPJ4
--The given question is incomplete, the complete question is
"Missed this? watch kcv: atomic theory; read section 2.3. you can click on the review link to access the section in your text. carbon and oxygen form both carbon monoxide and carbon dioxide. when samples of these are decomposed, the carbon monoxide produces 3.36 g of oxygen and 2.52 g of carbon, while the carbon dioxide produces 9.92 g of oxygen and 3.72 g of carbon. Calculate the atomic ratio of carbon to oxygen in carbon monoxide, and carbon dioxide."--
When a substance is heated ,the kinetic energy of its molecules
Answers
Answer:
When a substance is heated ,the kinetic energy of its molecules also increase.
Explanation:
K.E is directly proportional to T
1. Determine the molecular formula of an oxide of iron in which the mass of iron and oxygen are 69.9% and 30% respectively given that the molar mass of the oxide 159.898/mol, find the empirical and molecular formula.
2. a crystalline salt when heated becomes anhydrous and loses 51.2% of its weight the anhydrous salt analysis gave the percent composition as magnesium is equal to 20.0% and sulphur is equal to 26.66% and oxygen is equal to 53.33%.
3. In three moles of Ethane calculate the following
1. calculate number of carbon atoms.
2. number of moles of hydrogen atoms
3. number of molecules of Ethane.
Answers
1a. The empirical formula of the compound is Fe₂O₃
1b. The molecular formula of the compound is Fe₂O₃
2a. The molecular formula of the anhydrous salt is MgSO₄
2b. The formula of the crystalline salt is MgSO₄.7H₂O
3i. The number of mole of carbon atoms in the compound is 6 moles
3ii. The number of mole of hydrogen atoms in the compound is 18 moles
3iii. The number of molecules in 3 moles of ethane is 1.806×10²⁴ molecules
1a. How to determine the empirical formulaFe = 69.9%O = 30%Empirical formula =?Divide by their molar mass
Fe = 69.9 / 56 = 1.248
O = 30 / 16 = 1.875
Divide by the smallest
Fe = 1.248 / 1.248 = 1
O = 1.875 / 1.248 = 3/2
Multiply by 2 to express in whole number
Fe = 1 × 2 = 2
O = 3/2 × 2 = 3
Thus, the empirical formula of the compound is Fe₂O₃
1b. How to determine the molecular formulaEmpirical formula = Fe₂O₃Molar mass of compound = 159.89 g/molMolecular formula = ?Molecular formula = empirical × n = molar mass
[Fe₂O₃]n = 159.89
[(56×2) + (16×3)]n = 159.89
160n = 159.89
n = 159.89 / 160
n = 1
Molecular formula = [Fe₂O₃]n
Molecular formula = [Fe₂O₃] × 1
Molecular formula = Fe₂O₃
2a. How to determine the molecual formula of the anhydrous saltWe'll begin by calculating the empirical formula
Mg = 20.0% S = 26.66% O = 53.33%Empirical formula =?Divide by their molar mass
Mg = 20.0 / 24 = 0.83
S = 26.66 / 32 = 0.83
O = 53.33 / 16 = 3.33
Divide by the smallest
Mg = 0.83 / 0.83 = 1
S = 0.83 / 0.83 = 1
O = 3.33 / 0.83 = 4
Thus, the empirical formula of the anhydrous salt is MgSO₄
The molecular formula of the anhydrous salt can be obtained as follow:
Empirical formula = MgSO₄Molar mass of compound = 120 g/molMolecular formula = ?Molecular formula = empirical × n = molar mass
[MgSO₄]n = 120
[24 + 32 + (16×4)]n = 159.89
120n = 120
n = 120 / 120
n = 1
Molecular formula = [MgSO₄]n
Molecular formula = [MgSO₄] × 1
Molecular formula = MgSO₄
2b. How to determine the formula of the crystalline saltWater (H₂O) = 51.2%Anhydrous salt (MgSO₄) = 100 - 51.2 = 48.8%Formula of crystalline salt =?Divide by their molar mass
MgSO₄ = 48.8 / 120 = 0.4
H₂O = 51.2 / 18 = 2.8
Divide by the smallest
MgSO₄ = 0.4 / 0.4 = 1
H₂O = 2.8 / 0.4 = 7
Thus, the formula of the crystalline salt is MgSO₄.7H₂O
3i. How to determine the mole of carbon atoms in 3 moles of C₂H₆1 mole of C₂H₆ contains 2 moles of carbon atoms.
Therefore,
3 moles of C₂H₆ will contain = 3 × 2 = 6 moles of carbon atoms
3ii. How to determine the mole of hydrogen atoms in 3 moles of C₂H₆1 mole of C₂H₆ contains 6 moles of hydrogen atoms.
Therefore,
3 moles of C₂H₆ will contain = 3 × 6 = 18 moles of hydrogen atoms
3iii. How to determine the number of moleculesFrom Avogadro's hypothesis,
1 mole of ethane = 6.02×10²³ molecules
Therefore,
3 moles of ethane = 3 × 6.02×10²³ molecules
3 moles of ethane = 1.806×10²⁴ molecules
Learn more about empirical formula:
https://brainly.com/question/24297883
Learn more about Avogadro's number:
https://brainly.com/question/26141731
#SPJ1
Complete question
2. A crystalline salt when heated becomes anhydrous and loses 51.2% of its weight the anhydrous salt analysis gave the percent composition as magnesium is equal to 20.0% and sulphur is equal to 26.66% and oxygen is equal to 53.33%. Ccalculate the molecular formula of the anhydrous and the crystalline salt. The molecular weight of the anhydrous salt is 120
Why does the succession process struggle to regain its balance after certain environmental changes caused by human activities?
Answers
Answer:
Ecological succession is the process by which
the mix of species and habitat in an area changes over time.Gradually, these communities replace one another until a “climax community”—like a mature forest—is reached, or until a disturbance, like a fire, occurs. Ecological succession is a fundamental concept in ecology.
Explanation:
i hope it helps uh.!!
An iron object alloyed with cobalt rusts more quickly than a pure iron object. However, an iron object alloyed with manganese rusts less quickly than a pure iron object under the same conditions. This is true because: __________
a. cobalt is a stronger reducing agent than iron
b. iron is a stronger reducing agent than manganese
c. cobalt exhibits more metallic character than either iron or manganese in the iron-manganese alloy
Answers
Rusting is an electrochemical reaction. Iron rusts faster when alloyed with cobalt than when alloyed with manganese because, in the iron-manganese alloy, manganese is rendered the anode and iron is rendered the cathode
An alloy is a combination of two metals. There are various reasons for producing alloys such as greater tensile strength, corrosion resistance and improved aesthetic appearance.
When iron is alloyed with cobalt, the iron rusts faster than pure iron because iron is rendered the anode and cobalt is rendered the cathode. When the iron is alloyed with manganese, it rusts more slowly than pure iron because in the iron-manganese alloy, manganese is rendered the anode and iron is rendered the cathode.
Missing parts;
An iron object alloyed with cobalt rusts more quickly than a pure iron object. However, an iron object alloyed with manganese rusts less quickly than a pure iron object under the same conditions. This is true because
(1) cobalt is a stronger reducing agent than iron
(2) iron is a stronger reducing agent than manganese
(3) cobalt exhibits more metallic character than either iron or manganese
(4) in the iron-manganese alloy, manganese is rendered the anode and iron is rendered the cathode
(5) in the iron-cobalt alloy, cobalt is rendered the anode and iron is rendered the cathode
Learn more: https://brainly.com/question/14281129
2Mg + O2 --> 2MgO
write down the chemical formula of the product, which formed during the reaction
Chemical formula product:
Answers
Answer:
2MgO is the chemical formula of the product formed
I'm not too sure on this question: Give a balanced chemical equation for solid aluminum reacting with aqueous hydrogen monofluoride to produce aqueous aluminum fluoride and hydrogen gas.
Help would be much appreciated! :D
Answers
The balanced reaction equation is given as;
\(2Al + 6HF(aq) ------- > 2AlF_{3} (aq) + 3H_{2} (g)\)
What is the balanced chemical reaction equation?We know that a chemical reaction has to do with the interaction between the reactants which would lead to the formation of the products. We know that one of the rules that govern a chemical reaction is that a reaction equation would be said to be balanced if the number of the atoms of each of the elements on the reactants side is the same as the number of the atoms on the products side.
The balanced reaction equation of the reaction between solid aluminum reacting with aqueous hydrogen monofluoride to produce aqueous aluminum fluoride and hydrogen gas has been shown above.
Learn more about reaction equation:https://brainly.com/question/3588292
#SPJ1
Help what's the answer??

Answers
The mass of the excess reagent (iron) that remains after the reaction is complete is 12.1 grams.
The maximum mass of Iron(III) oxide that can be formed is 74.8 grams.
The formula for the limiting reactant is O₂, which is oxygen gas.
Calculate the number of moles of each reactant:
Number of moles of iron = 38.2 g / 55.845 g/mol = 0.683 moles
Number of moles of oxygen = 14.9 g / 32.00 g/mol = 0.466 moles
The balanced equation shows that the stoichiometric ratio between iron and oxygen is 4:3. Therefore, the mole ratio of iron to oxygen is 4:3.
Since the mole ratio of iron to oxygen is greater than 4:3, it means that there is an excess of iron and oxygen is the limiting reactant. So we need to use the number of moles of oxygen to calculate the maximum mass of Iron(III) oxide that can be formed.
The balanced equation shows that 1 mole of iron reacts with 1 mole of oxygen to produce 1 mole of Iron(III) oxide. The molar mass of Iron(III) oxide is 159.69 g/mol.
Number of moles of Iron(III) oxide = 0.466 moles (which is equal to the number of moles of oxygen)
The maximum mass of Iron(III) oxide = Number of moles of Iron(III) oxide x molar mass of Iron(III) oxide
Maximum mass of Iron(III) oxide = 0.466 moles x 159.69 g/mol = 74.8 grams
The maximum mass of Iron(III) oxide that can be formed is 74.8 grams.
The formula for the limiting reactant is O₂, which is oxygen gas.
To calculate the mass of the excess reagent (iron) that remains after the reaction is complete, we need to first calculate the mass of iron that reacted with the oxygen:
Mass of iron reacted = 0.466 moles x 55.845 g/mol = 26.1 grams
The initial mass of iron was 38.2 grams, so the mass of excess iron that remains after the reaction is complete is:
Mass of excess iron = initial mass of iron - a mass of iron reacted
Mass of excess iron = 38.2 g - 26.1 g = 12.1 grams
The mass of the excess reagent (iron) that remains after the reaction is complete is 12.1 grams.
learn more about limiting reagent here
https://brainly.com/question/26905271
#SPJ1
Write the balanced net ionic equation for the reactions that occur when the given aqueous solutions are mixed. Include the physical states. A. Silver nitrate, agno3 , and magnesium bromide, mgbr2.
Answers
A silver precipitate is created when silver nitrate, AgNO3, and magnesium bromide, MgBr2, are combined in aqueous solutions.Create the net ionic equation as well as the balanced formula equation for this reaction.
When AgNO3 and MgCl2 are combined, does a solid result?The end products of the reaction between aqueous systems of magnesium chloride (MgCl2) and silver nitrate (AgNO3) are solid silver chloride and aqueous magnesium nitrate.
Which one of the following results in an AgCl and AgNO3 precipitate?The right response is (CH3)3C-Cl.The most reliable 3° carbocation is formed by tert-butyl chloride, or (CH3)3C - Cl.As a result, it will immediately produce the white precipitate of AgCl in AgNO3 solution.
To know more about ionic equation visit:
https://brainly.com/question/15467502
#SPJ4
Diffusion and Organelle Retake Activity
(please finish in 5-9 minutes)
Question:
Fill in the blank
Water loves _____ and ____.
This means water will
move ______ the direction of the salt or sugar.
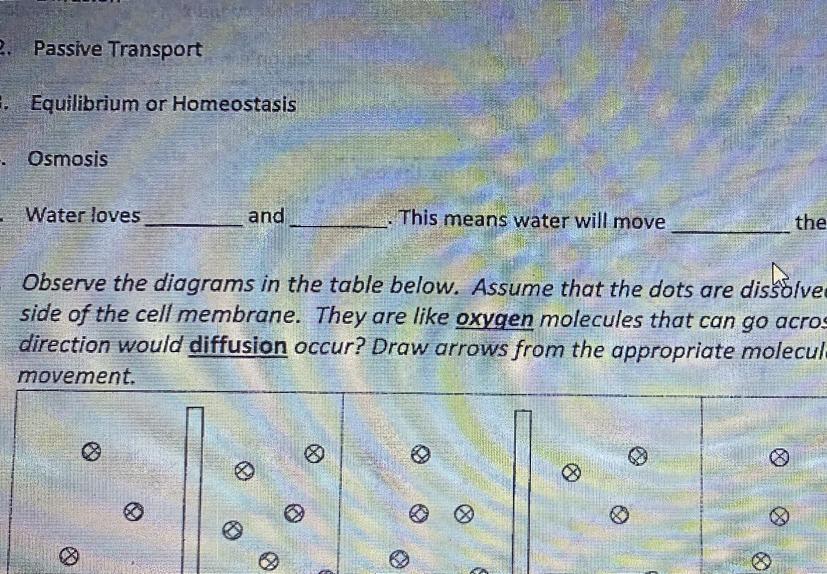
Answers
Answer:
wind and soil? north?
Explanation:
When bonds are (broken/formed) there is a positive energy change.
Answers
Answer: Hello i am confused are you asking a question?
Explanation:
A reaction has an enthalpy change of − 71 kJ mol − 1 and an entropy change of − 58 J K − 1 mol − 1 . At what temperature does this exothermic reaction cease to be spontaneous?
Answers
To determine the temperature at which an exothermic reaction ceases to be spontaneous, we need to calculate the Gibbs free energy change (ΔG) and use the equation ΔG = ΔH - TΔS.
Given that ΔH = -71 kJ/mol and ΔS = -58 J/K·mol, we can calculate ΔG at different temperatures to determine the temperature at which the reaction becomes non-spontaneous.
At a temperature of 0 K, ΔG = ΔH, since TΔS = 0. Thus, ΔG = -71 kJ/mol.
As the temperature increases, TΔS becomes more negative, which means that ΔG becomes more negative, making the reaction more spontaneous.
At a certain temperature, however, ΔG will become positive, which means that the reaction is no longer spontaneous and will not proceed on its own. This temperature can be found by rearranging the equation ΔG = ΔH - TΔS to T = ΔH / ΔS, and substituting the known values for ΔH and ΔS:
T = ΔH / ΔS = -71 kJ/mol / (-58 J/K·mol) = 1230 K
So, the reaction will cease to be spontaneous at a temperature of approximately 1230 K.
PLEASE HELP!!!!!
Calcium hydroxide is _____.
an acid
a base
a neutral
Answers
A student proposed the Bohr model below for sodium (Na). Is this student’s model correct? Justify your answer

Answers
The Bohr model is a representation of the electronic configuration of the atom. According to this model, each energy level can hold a certain number of electrons. In the first energy there can only be 2 electrons, in the second and the following energy levels there can be a maximum of 8 electrons.
Sodium, Na which has 11 electrons in total so in the first level it will have two electrons, in the second level it will have 8 electrons and in the third level it will have the missing electron.
In the model proposed by the student, the electrons are represented in blue. The model proposed by the student is incorrect.
We see that in the second energy level he drew 9 electrons, this is incorrect since from the second energy level there can only be 8 electrons, the remaining electron must be located in the third energy level.