what is the heat of a reaction, in joules, with a total reaction mixture volume of 67.1 ml if the reaction causes a temperature change of 6.7 oc in a calorimeter? assume that the reaction mixture has a density of 1.00 g/ml and a specific heat of 4.184 j/g-oc. the calorimeter has a heat capacity of 10.0 j/oc.
Answers
We can calculate the heat of the reaction using the equation above:
q = -C_cal * ΔT
q = -10.0 j/oc * 6.7 oc
q = -67.0 j
The heat of a reaction can be calculated using the equation:
q = -C_cal * ΔT
where q is the heat absorbed or released by the reaction, C_cal is the heat capacity of the calorimeter, and ΔT is the temperature change.
First, we need to calculate the mass of the reaction mixture, which can be found using the density and volume:
mass = density * volume
mass = 1.00 g/ml * 67.1 ml
mass = 67.1 g
Next, we need to calculate the total heat capacity of the reaction mixture and calorimeter:
C_total = mass * specific heat + C_cal
C_total = 67.1 g * 4.184 j/g-oc + 10.0 j/oc
C_total = 287.3 j/oc
Finally, we can calculate the heat of the reaction using the equation above:
q = -C_cal * ΔT
q = -10.0 j/oc * 6.7 oc
q = -67.0 j
To know more about calorimeter here
https://brainly.com/question/31029004
#SPJ4
Related Questions
if you react 25.0g of Cu with 25.0g of AlCl3 in the following reaction 3Cy + 2AlCl3 -> 3CuCl2 + 2Al
a. find the excess and limiting reactants
b. calculate the mass of leftover reactant
Answers
a. AlCl₃ ⇒ limiting reactant(smaller ratio)
Cu ⇒ excess reactant
b. the mass of leftover reactant : 7.207 g
Further explanationGiven
25 g Cu
25 g AlCl3
Required
a. the excess and limiting reactants
b. the mass of leftover reactant
Solution
Reaction
3Cu + 2AlCl₃ ⇒ 3CuCl₂ + 2Al
mol Cu(Ar = 63.5 g/mol) :
mol = mass : Mw
mol = 25 : 63.5
mol = 0.394
mol AlCl3(MW=133,34 g/mol) :
mol = 25 : 133,34 g/mol
mol = 0.187
mol ratio to reaction coefficient Cu : AlCl₃ =
\(\tt \dfrac{0.394}{3}\div \dfrac{0.187}{2}=0.131\div 0.093\)
AlCl₃ ⇒ limiting reactant(smaller ratio)
Cu ⇒ excess reactant
b. the mass of leftover reactant :
mol Cu = 3/2 x 0.187 = 0.2805
mol left = 0.394 - 0.2805 = 0.1135
mass = 0.1135 x 63.5 = 7.207 g
pls answer question will mark brainliset tyty

Answers
Answer:
Forests are a renewable resource
Answer: B. Forests
Explanation:
This is because, as part of its biological cycle, carbon is taken up by trees and becomes forest biomass that eventually dies, decays, and releases carbon that is in turn taken back up by renewed forest growth.
in a properly constructed lewis dot structure, how many pairs of electrons are there in an atom of nitrogen?
Answers
A nitrogen atom has five valence electrons, which can be shown as one pair and three single electrons.
What is lewis dot structure ?Lewis structures, also known as Lewis dot formulas, Lewis dot structures, electron dot structures, or Lewis electron dot structures, are diagrams that display the interatomic interactions as well as any lone pairs of electrons that may be present in a molecule.
Two double bonds exist between the atoms of carbon and oxygen in the Lewis structure of CO2. Each oxygen atom must form bonds with four distinct carbon atoms, according to the octet rule.Having five valence electrons, nitrogen. 4 electrons are arranged on either side of an N to represent the Lewis structure. Next, one of the four electrons is paired with the fifth electron. There are now 3 unpaired electrons left.Learn more about Lewis dot structure here:
https://brainly.com/question/20300458
#SPJ4
Convert 2.76 atm to mmHg
Answers
Answer:2097.5998675091255
Explanation:
Why do nuclear fusions release energy?
Answers
Answer:
The release of energy with the fusion of light elements is due to the interplay of two opposing forces: the nuclear force, which combines together protons and neutrons, and the Coulomb force, which causes protons to repel each other.
Question 8
I need help

Answers
Answer:A
Explanation: Since the boiling point is 212. As salt keeps getting added the boiling temperature keeps going up. Meaning it will boil at a high temp.
3.) All matter has both physical and chemical properties. A physical property is one that does not change the chemical nature of matter. Which of these choices is a physical property?
A.) height
B.) flammability
C.) ability to rust
D.) reaction with water
Answers
Answer:
D
Explanation:
It would be D because you are observing the reaction and don’t change anything
What element does Chlorine go in?
- metals
-not metals
-Metalloids
Answers
Answer:
non of them it is
halogen elements
in which shell duplet rule is applied, why?
Answers
Answer:
In first shell duplet rule is applied because first shell contains maximum 2 electrons
Explanation:
A 20. 0 g lead ball is heated in a Bunsen burner to 705 degrees celsius. It is then dropped into a 500. 0 g water bath. What is the initial temperature of the water if the final temperature is 35 degrees celsius? The C of lead is 0. 13 J/g degrees C.
[ Remember: Ch2o = 4. 18 J/g degrees celsius]
Answers
The initial temperature of the water is 25.8 °C. As a result, the lead ball loses heat rapidly when it is placed in the water bath, causing the water temperature to increase significantly.
What is Temperature?
Temperature is a measure of the average kinetic energy of the particles in a substance. It is a physical quantity that describes how hot or cold an object is. Temperature is usually measured using a thermometer and is commonly expressed in units such as degrees Celsius (°C), Fahrenheit (°F), or Kelvin (K).
The energy gained by the water can also be calculated using the formula:
Q = mcΔT
where Q is the energy gained (in joules), m is the mass of the water (in grams), c is the specific heat capacity of water (in J/g°C), and ΔT is the change in temperature of the water (in °C).
We can calculate Q as follows:
Q = (500.0 g)(4.184 J/g°C)(35°C - T)
where T is the initial temperature of the water.
Since the energy lost by the lead ball is equal to the energy gained by the water, we can set these two equations equal to each other and solve for T:
(20.0 g)(0.13 J/g°C)(705°C - T) = (500.0 g)(4.184 J/g°C)(35°C - T)
Simplifying and solving for T gives:
T = 25.8°C
Therefore, the initial temperature of the water is 25.8 °C.
To know more about Temperature, visit;
https://brainly.com/question/26866637
#SPJ4
............................
Answers
Answer:
............................
Explanation:
........................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................
Write a strong claim about the question:
What shape are cells?
( i don't really need a long answer)
Answers
Answer:
Usually, the cells are round, elongated or spherical. There are also some cells which are long and pointed on both the ends. Such cells exhibit spindle shape. In some cases, the cells are very long.
Explanation:
Hope I helped! Brainliest plz!
The information below pives PH values of solutions , w,XYZ
Solution
Pl values
2
6.5
W
Y
z
14
4.5
(a) Which solution is likely to be:
(i) Calcium hydroxide?
(1) Rain water?
Answers
Answer:
The calcium hydroxide solution will likely have the pH of 14 (very basic) while rain water will likely have a pH if 6.5 (slightly acidic).
Classify the statements based on whether they describe a weak or strong mobile phase in affinity chromatography. a. referred to as the elution buffer b. referred to as the application buffer c. mimics the pH, ionic strength, and polarity of the affinity ligand's natural environment d. readily removes the analyte from the affinity ligand e. a competing agent is used to displace the analyte from the affinity ligand f. The pH, ionic strength, or polarity is changed to decrease the association equilibrium constant between the analyte and affinity ligand g. promotes strong binding between the analyte and affinity ligand
Answers
Statements about weak and strong mobile phase in affinity chromatography include,
Weak mobile phase:
(d) Enables efficient dissociation of the analyte from the affinity ligand.
(e) Utilizes a competing agent to displace the analyte from the affinity ligand.
(f) Involves modifying the pH, ionic strength, or polarity to reduce the association equilibrium constant between the analyte and affinity ligand.
Strong mobile phase:
(a) Referred to as the elution buffer, it facilitates the release of the analyte from the affinity ligand.
(b) Known as the application buffer, it promotes the initial binding of the analyte to the affinity ligand.
(c) Mimics the pH, ionic strength, and polarity of the affinity ligand's natural environment, enhancing favorable interactions.
(g) Fosters robust binding between the analyte and affinity ligand, ensuring strong affinity interactions.
In affinity chromatography, the choice between a weak or strong mobile phase depends on the desired outcome.
A weak mobile phase is employed to remove the analyte from the affinity ligand or decrease binding strength, achieved through efficient dissociation, displacement, or modification of relevant factors.
Conversely, a strong mobile phase is used to facilitate binding interactions, encompassing initial application, mimicking the natural environment, and promoting robust binding between the analyte and affinity ligand.
Learn more about affinity chromatography at: https://brainly.com/question/14397727
#SPJ11
The density of acetic acid is 1.05 g/mL. What is the volume of 327 g of acetic acid
Answers
Answer:
\(311.43\ \text{mL}\)
Explanation:
\(\rho\) = Density of acetic acid = \(1.05\ \text{g/mL}\)
\(m\) = Mass of acetic acid = \(327\ \text{g}\)
\(V\) = Volume
Density is given by
\(\rho=\dfrac{m}{V}\\\Rightarrow V=\dfrac{m}{\rho}\\\Rightarrow V=\dfrac{327}{1.05}\\\Rightarrow V=311.43\ \text{mL}\)
The volume of acetic acid is \(311.43\ \text{mL}\).
3H2SO4 + 2B(OH)3 → B2(SO4)3 + 6H₂O
What is the mole ratio between
water and sulfuric acid?
[ ? ] mol H2O
mol H2SO4
Answers
The mole ratio between the sulfuric acid and the water is 3:6.
What is the mole ratio?We know that the mole ratio has to do with the ratio of the molar coefficients of the reactants and the products. When we are talking about the molar coefficients, we are talking about the numbers that are written before the symbols of the compounds.
We can now see that there are six moles of the water and there are three moles of the sulfuric acid. The sulfuric acid is the compound that has the symbol \(H_{2} SO_{4}\).
Learn more about the mole ratio:https://brainly.com/question/15288923
#SPJ1
Answer:
Explanation:
its 6:3 not 3:6 not trying to be rude but its the other way around!!! :)
What is a superconductor?
A. A conductor that operates at room temperature
B. A conductor that allows electricity to flow easily
C. A conductor that conducts electricity faster than common metals
D. A conductor that allows electricity to flow through nonmetal solids

Answers
Answer:
its B
Explanation:
a p e x
Answer:
the answer is b. allows electricity to flow easily
help me with this ?
i need to git this done for school
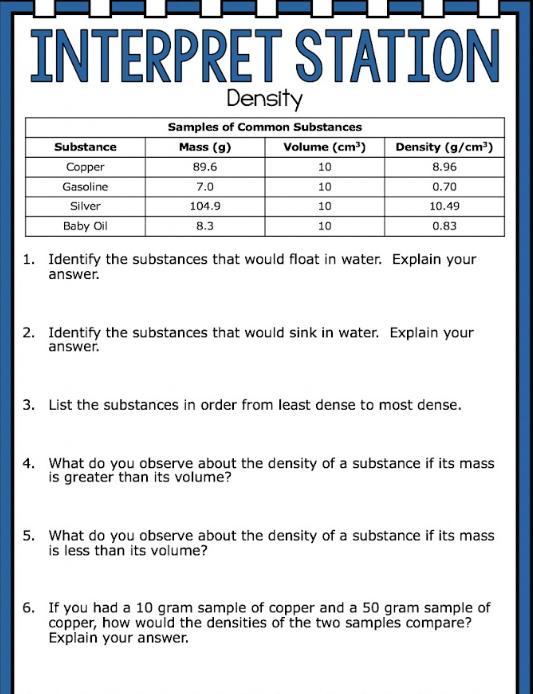
Answers
1) Gasoline and baby oil will float in water
2) Copper and silver will sink in water
3) Silver, Copper, Baby oil Gasoline
4) If the mass is less than volume the density will be less than one
5) If the mass is greater than the volume the density is not less than 1
6) The densities of the substances would be the same.
What is the density?Density is a physical property of matter that measures how much mass is contained in a given volume. It is calculated as the ratio of mass to volume, and is typically expressed in units of grams per cubic centimeter (g/cm³) or kilograms per cubic meter (kg/m³).
The formula for calculating density is:
Density = Mass / Volume
Where Mass is the amount of matter in an object or substance, and Volume is the amount of space that it occupies. Density is an intensive property, which means that it is independent of the amount of substance being measured. For example, the density of a material will be the same regardless of whether you measure it in grams or kilograms.
Learn more about density:https://brainly.com/question/29775886
#SPJ1
1.In the electrolysis of molten LiBr, which product forms at the anode? 1. Li(l) 2. Br2(g) 3. H2(g) 4. O2(g)
2.In the electrolysis of molten FeI3, which product forms at the anode? 1. Fe(l) 2. O2(g) 3. H2(g) 4. I2(g)
3.Hydrogen can be prepared by suitable electrolysis of aqueous calcium salts 1. True 2. False
4.Hydrogen can be prepared by suitable electrolysis of aqueous silver salts 1. False 2. True
5.What product(s) forms at the cathode in the electrolysis of an aqueous solution of NaCl? 1. O2 and H+ 2. Na 3. Cl2 4. H2 and OH-
Answers
\(Br_2\)(g) (option 2) is the byproduct created at the anode during the electrolysis of molten LiBr. \(I_2\)(g) (option 4) is the end result of the electrolysis of molten\(FeI_3\) at the anode. True.
In the chemical process of electrolysis, a substance is broken down into its individual elements or ions. It involves causing chemical processes to take place at the electrodes by passing an electric current through an electrolyte, often a liquid or solution containing ions. Anode and cathode are the terms used to describe the electrodes linked to the positive and negative terminals of a power source, respectively.
1) \(Br_2\)(g) (option 2) is the byproduct created at the anode during the electrolysis of molten LiBr.
2) \(I_2\)(g) (option 4) is the end result of the electrolysis of molten\(FeI_3\) at the anode.
3) True. Through the proper electrolysis of aqueous calcium salts, hydrogen can be produced.
4)False. The appropriate electrolysis of aqueous silver salts cannot produce hydrogen.
5) \(H_2\) and \(OH^-\) are the product(s) generated at the cathode during the electrolysis of a NaCl aqueous solution (option 4).
To know more about electrolysis, here:
https://brainly.com/question/12994141
#SPJ4
based on the calculations performed in this experiment, would the same mass of a solute with a significantly higher molar mass have a larger or smaller effect on the boiling point elevation?
Answers
Based on the calculations performed in this experiment, the same mass of a solute with a significantly higher molar mass would have a larger effect on the boiling point elevation. As a result, the same mass of a solute with a higher molar mass will have a greater effect on the boiling point elevation.
Boiling point elevation is a thermodynamic phenomenon that occurs when the boiling point of a solvent (a substance that dissolves a solute to create a solution) is increased by adding another substance, the solute, to it. When a solute is added to a solvent, it lowers the freezing point and raises the boiling point of the solvent, which is known as the boiling point elevation.The formula for boiling point elevation is: ∆Tb = Kbm
Here, ∆Tb is the boiling point elevation, Kb is the molal boiling point elevation constant, and m is the molality of the solution. To understand this, let us take an example: Suppose a solution containing 1.0 mol of sodium chloride (NaCl) is dissolved in 1.0 kg of water. The molality of the solution is 1.0 mol / 1.0 kg = 1.0 m. In addition, the Kb for water is 0.51 °C/molal, which means that the boiling point elevation is 0.51 °C when the molality of the solution is 1.0 mol/kg.So, the boiling point of the solution will be raised by 0.51 °C, which can be calculated using the above formula.Calculation performed in this experiment:Boiling point elevation = ΔTb = Kb . mTherefore, based on the above formula, the boiling point elevation is directly proportional to the molality of the solution, which, in turn, is directly proportional to the number of moles of solute in the solution. Furthermore, the number of moles of solute is proportional to the mass of the solute (in grams) divided by its molar mass (in grams/mol).So, if a solute with a significantly higher molar mass is added to the solvent, it will have a larger effect on the boiling point elevation. As a result, the same mass of a solute with a higher molar mass will have a greater effect on the boiling point elevation.
To know more about boiling point elevation visit:
https://brainly.com/question/30641033
#SPJ11
Assuming the salt is sodium chloride, what is the approximate molar concentration of salt in ocean water if the density of ocean water is 1.028 kg/l?
Answers
Molar concentration of Sodium chloride (NaCl) means the number of moles of sodium chloride present in 1L of water.
Molar mass of NaCl = Molar mass of Na + Molar mass of Cl
Molar mass of NaCl = 23 + 35.5
Molar mass of NaCl = 58.5g
∴ 1L of water contains 55.55 moles of water. Hence,
Mass of ocean water = 55.55 × 18 = 999.9g
Since, 1L of ocean water has a mass of 999.9g of which 3.5% is the salt.
hence, (999.9) (0.035) = 34.99g
So, No. of moles of NaCl = Mass of NaCl / 58g NaCl per mol
No. of moles of NaCl = 34.99/58.5
No. of moles of NaCl = 0.59 moles
So, Molar concentration or Molarity would be 0.59 moles present in 1L of solution.
Hence, Molarity or Molar Concentration = Moles of NaCl/ Volume of solution (in L)
Molarity = 0.59/1L
⇒ Molarity = 0.59M
Hence, the molarity is 0.59M
Learn more about Molarity here, https://brainly.com/question/8732513
#SPJ1
while hydrogen, helium, water, and ammonia can produce the white coloration of jupiter's zones, the brownish color of the belts requires more complex chemistry. T/F
Answers
Jupiter's atmosphere is composed mostly of hydrogen and helium, with small amounts of other gases such as water and ammonia.
The white coloration in the zones of Jupiter is thought to be produced by these gases reflecting sunlight. However, the brownish color of the belts is not as easily explained by simple reflection of sunlight.
The belts have a more complex chemistry, with various compounds such as ammonia, methane, and sulfur mixing with the hydrogen and helium.
These compounds absorb different wavelengths of light, which can produce the brownish coloration. Additionally, the belts have stronger winds and more turbulence than the zones, which can also affect the color.
Overall, while simple gases like hydrogen, helium, water, and ammonia play a role in Jupiter's coloration, the belts require more complex chemistry to produce their distinctive brown hue.
To know more about ammonia refer here
https://brainly.com/question/29519032#
#SPJ11
A hydrogen atom makes a downward transition from the n=19 state to the n=5 state, Find the wavelength of the emitted photon. 2.45μm 2.94μm 1.47μμm 1.96μμm
Answers
The wavelength of the emitted photon is approximately 2.44 μm.
The wavelength of the emitted photon can be determined using the Rydberg formula as follow
\(\frac{1}{\lambda}=R_H\left(\frac{1}{n_1^2}-\frac{1}{n_2^2}\right)\)
where: lambda is the wavelength of the emitted photon,
\(R_H=1.0974\times10^7\text{m}^{-1} ,$n_1=19 n_2=5 :\frac{1}\)
\({\lambda}=R_H\left(\frac{1}{19^2}-\frac{1}{5^2}\right) \frac{1}\\\\\\{lambda}=1.0974\times10^7\text{m}^{-1}\left(\frac{1}{361}-\frac{1}{25}\right) \frac{1}\\{\lambda}=1.0974\times10^7\text{m}^{-1}\left(0.002709-0.04\right) \frac{1}\\{\lambda}=1.0974\times10^7\text{m}^{-1}\times(-0.037291) \frac{1}{\lambda}=-409446.34\text{m}^{-1} \lambda=-\frac{1}{409446.34\text{m}^{-1}}=2.44\times10^{-6}\text{m}\)
Therefore, the wavelength of the emitted photon is approximately 2.44 μm.
Rounded to two decimal places, this value is equal to 2.45 μm. Thus, the correct option is A) 2.45μm.
Learn more about wavelenght with the given link,
https://brainly.com/question/10750459
#SPJ11
-8 + 3u = 3u - 8
no solution
one solution
infinitely many solutions
Answers
Answer: infinitely many solutions
Explanation: since this equation is the same on both sides, any value that is plugged in for "u" will equal the same amount on both sides.
example) -8 + 3(1) = 3(1) - 8
-8 +3 = 3 + -8
-5 = -5
Help ASAP wil give brainlist pls

Answers
2A3B ---- 4A2 + B2
It is a balancing equation right?
F. How many centigrams are in 253,000 picograms?
Plz show work
Answers
The answer is 2.53e-5, I unfortunately don't know how you would really show the work other than showing the division.
A 10,0-L cylinder of gas is stored at room temperature (20.0°C) and a pressure of 1800 psi. If the gas is
transferred to a 6.0-L cylinder, at what temperature in CELCIUS would it have to be stored in order for the
pressure to remain at 1800 psí? Reminder, convert your temperature to Kelvin before you begin the problem.
(Please put units)
Answers
Considering the Charles' law, the gas would have a temperature of -109.2 C.
Charles' lawFinally, Charles' law establishes the relationship between the volume and temperature of a gas sample at constant pressure. This law says that the volume is directly proportional to the temperature of the gas. That is, if the temperature increases, the volume of the gas increases, while if the temperature of the gas decreases, the volume decreases.
Charles' law is expressed mathematically as:
\(\frac{V}{T} =k\)
If you want to study two different states, an initial state 1 and a final state 2, the following is true:
\(\frac{V1}{T1} =\frac{V2}{T2}\)
Temperature of the gas in this caseIn this case, you know:
P1= 1800 psiV1= 10 LT1= 20 C= 293 K (being 0 C= 273 K)P2= 1800 psiV2= 6 LT2= ?You can see that the pressure remains constant, so you can apply Charles's law.
Replacing in the Charles's law:
\(\frac{10 L}{293 K} =\frac{6 L}{T2}\)
Solving:
\(\frac{10 L}{293 K} T2=6 L\)
\(T2=\frac{6 L}{\frac{10 L}{293 K} }\)
T2=163.8 K= -109.2 C
The gas would have a temperature of -109.2 C.
Learn more about Charles's law:
https://brainly.com/question/4147359?referrer=searchResults
To 225 mL of a 0.80M solution of KI, a student adds enough water to make 1.0 L of a more dilute KI solution. What is the molarity of the new solution
Answers
M1V1 = M2V2
where M1 is the initial molarity, V1 is the initial volume, M2 is the final molarity, and V2 is the final volume.
In this case, we have:
M1 = 0.80 M
V1 = 225 mL = 0.225 L
V2 = 1.0 L
We can rearrange the equation to solve for M2:
M2 = M1V1 / V2
Plugging in the values, we get:
M2 = (0.80 M)(0.225 L) / 1.0 L
Simplifying this equation, we get:
M2 = 0.18 M
Therefore, the molarity of the new KI solution is 0.18 M.
This image shows electronegativities of elements on the periodic table. Based on this information, which element is most likely to form a strong ionic bond and take on electrons?
(1 point)
helium (He)
cesium (Cs)
fluorine (F)
hydrogen (H)
Answers
Answer:
The answer is C. Fluorine.
calculate the ph of a 0.150 m piperidine (c5h10nh) solution (kb = 1.3x10-3).
Answers
The pH of a 0.150 M piperidine solution (C5H10NH) with a Kb of 1.3x10^-3 is 11.72. This indicates that the solution is basic.
To calculate the pH of a 0.150 M piperidine (C5H10NH) solution with Kb = 1.3 x 10^-3, we'll first determine the pOH and then find the pH. Piperidine is a weak base and will undergo an equilibrium reaction with water:
C5H10NH + H2O ↔ C5H10NH2+ + OH-
We can use the Kb expression:
Kb = [C5H10NH2+][OH-] / [C5H10NH]
Since the initial concentration of piperidine is 0.150 M, we'll assume x mol/L of it reacts to form C5H10NH2+ and OH- ions. The equilibrium concentrations will be:
[C5H10NH] = 0.150 - x
[C5H10NH2+] = x
[OH-] = x
Now, substitute these values into the Kb expression:
1.3 x 10^-3 = (x)(x) / (0.150 - x)
Solve for x to find the concentration of OH- ions:
x ≈ 0.0053 M
Now, calculate the pOH:
pOH = -log10[OH-] = -log10(0.0053) ≈ 2.28
Finally, find the pH using the relationship:
pH + pOH = 14
pH = 14 - 2.28 ≈ 11.72
So, the pH of the 0.150 M piperidine solution is approximately 11.72.
To know more about piperidine visit:
https://brainly.com/question/31315744
#SPJ11