What is the final concentration (M) of a solution prepared by diluting 50.0 mL of a solution to a volume of
Answers
D) 0.600 is the final concentration of the solution of KCl.
V1 = 50.0 mL.
Related Questions
how many molecules are in 4.62 moles of nitric acid
Answers
The number of molecules in 4.62 moles nitric acid has been 27.82 × 10²³.
It's likely that the common person has had no notion how many molecules make up 4.62 milligrams of hydrogen peroxide, but that's good! By the end of this article, you will be able to confidently respond to that question since you will having studied everything you needed concerning moles and molecules.
The moles have been described as the mass of the compound with respect to the molar mass. According to the Avogadro law, the number of molecules present in a mole of sample has been equivalent to the Avogadro number.
Therefore,
The Avogadro number has been a constant quantity with the value of .
6.023 × 10²³
The Avogadro law has been given as:
1 mole = 6.023 × 10²³ moles
Calculating for 4.62 moles nitric acid as:
4.60 moles = 6.023 × 10²³ × 4.62 molecules
⇒ 4.60 moles = 27.82 × 10²³ molecules.
The number of molecules in 4.62 moles nitric acid has been 27.82 × 10²³
Consequently, there are 27.828 x 10′′23 nitric acid molecules in 4.62 moles of nitric acid. This article is not simply concerning Avogadro's number. It turns out that these mass of the particles impacts how many particles are present in a mole of a substance So.
Complete Question:
How many molecules are in 4.62 moles of nitric acid (HNO3)?
To know more about Molecules click here
brainly.com/question/19922822
#SPJ4
a chemist adds 1.80 l of a 3.27 x 10^-5 mm silver oxide ag o solution to a reaction flask calculate the micromoles of silver oxide the chemist has added to the flask
Answers
A chemist adds 1.80 l of a 3.27 x 10⁻⁵ mm silver oxide ag o solution to a reaction flask, the micromoles of silver oxide the chemist has added to the flask is 58.86 micromoles.
To calculate the micromoles of silver oxide added to the flask, we need to use the given information: the volume of the solution and the concentration of silver oxide.
Volume of the solution = 1.80 L
Concentration of silver oxide = 3.27 x 10⁻⁵ mmol/L
1 liter is equal to 1000 milliliters, so we can convert the volume as follows:
1.80 L × 1000 mL/L = 1800 mL
Now we have the volume of the solution in milliliters.
To convert millimoles to micromoles, we multiply by a factor of 1000. Since we are converting from mmol/L to µmol/mL, we can convert the concentration as follows:
3.27 x 10⁻⁵ mmol/L = 3.27 x 10⁻² µmol/mL
To calculate the micromoles of silver oxide added to the flask, we multiply the volume in milliliters by the concentration in micromoles per milliliter.
1800 mL × 3.27 x 10⁻² µmol/mL = 58.86 µmol
Therefore, the chemist has added approximately 58.86 micromoles of silver oxide to the flask.
To know more about silver oxide here
https://brainly.com/question/3994710
#SPJ4
How many joules are required to melt 48 grams of Ammonia (NH3)?
The heat of fusion of ammonia is 5.66 kJ/mole. Round to the
appropriate amount of significant figures
Answers
The amount of energy required to melt 48 grams of ammonia is approximately 15.97 kJ
To calculate the amount of energy required to melt 48 grams of ammonia (NH₃), we need to use the heat of fusion of ammonia, which is given as 5.66 kJ/mole.
First, we need to calculate the number of moles of ammonia in 48 grams;
Number of moles = mass/molar mass
The molar mass of ammonia (NH3) is;
Nitrogen (N) = 14.01 g/mol
Hydrogen (H) = 1.01 g/mol
Molar mass of NH₃ = (14.01 g/mol) + 3(1.01 g/mol)
= 17.03 g/mol
Number of moles of NH₃ = 48 g / 17.03 g/mol = 2.82 moles
Next, we can use the heat of fusion of ammonia to calculate the amount of energy required to melt 2.82 moles of ammonia:
Energy = moles × heat of fusion
Energy = 2.82 moles × 5.66 kJ/mole
= 15.97 kJ
Therefore, the amount of energy required is 15.97 kJ
To know more about heat of fusion here
https://brainly.com/question/14053504
#SPJ1
a 1.25 l sample of an unknown diatomic gas, measured at stp, has a mass of 3.96 grams. what is the identity of the gas?
Answers
The identity of the unknown diatomic gas is chlorine (Cl₂).
The molar mass of a diatomic gas can be determined using its mass and volume at standard temperature and pressure (STP) in order to identify it. Here, the volume and mass of an unknown diatomic gas measured at STP are given.
The ideal gas law is PV = nRT, where P is pressure, V is volume, n is the number of moles, R is the gas constant, and T is the temperature in Kelvin. At STP, P = 1 atm and T = 273 K. Thus, we can simplify the ideal gas law to:
V = nRT/P
V = 1.25 L.
R = 8.31 J/(mol K).
1 L = 0.001 m³
1.25 L = 0.00125 m³
n = PV/RT
= (1 atm × 0.00125 m³) / (8.31 J/(mol K) × 273 K)
≈ 5.0 × 10⁻⁴ mol
Molar mass (MM) is defined as the mass of one mole of a substance. It is given by:
MM = mass/moles
The mass of the gas is given as 3.96 grams.
MM = 3.96 g / 5.0 × 10⁻⁴ mol
≈ 7.92 × 10³ g/mol
The molar mass of the gas is 7.92 × 10³ g/mol. We can compare this to the molar masses of diatomic gases listed in a periodic table. The only diatomic gas with a molar mass close to this value is Cl₂, which has a molar mass of 7.0 × 10³ g/mol.
Therefore, the identity of the unknown diatomic gas is chlorine (Cl₂).
To know more about topic Diatomic gas here: https://brainly.com/question/32798017
#SPJ11
ow do the z, n, and a values compare for each pair of atoms? (a) 3 h 1 and 3 he 2 (b) 14 c 6 and 15 n 7 (c) 19 f 9 and 18 f 9
Answers
Let's compare the Z, N, and A values for each pair of atoms:
(a) 3H1 and 3He2:
- 3H1: Z=1, N=2, A=3
- 3He2: Z=2, N=1, A=3
The Z and N values are reversed, while the A values are equal.
(b) 14C6 and 15N7:
- 14C6: Z=6, N=8, A=14
- 15N7: Z=7, N=8, A=15
Z values differ by 1, N values are equal, and A values differ by 1.
(c) 19F9 and 18F9:
- 19F9: Z=9, N=10, A=19
- 18F9: Z=9, N=9, A=18
Z values are equal, N values differ by 1, and A values differ by 1.
For the first pair of atoms, 3 H 1 and 3 He 2, the z value (atomic number) is different as hydrogen has 1 proton while helium has 2. The n value (number of neutrons) is also different as helium has one more neutron than hydrogen. However, the a value (mass number) is the same for both, which is 3.
For the second pair, 14 C 6 and 15 N 7, the z value is different as carbon has 6 protons while nitrogen has 7. The n value is also different as nitrogen has one more neutron than carbon. Therefore, the a value is different for both, with carbon having an a value of 14 and nitrogen having an a value of 15.
Finally, for the third pair, 19 F 9 and 18 F 9, the z value is different as fluorine has 9 protons while the other fluorine atom has 1 less proton. However, the n value is the same for both, which is 10. Therefore, the a value is different for both, with the first fluorine atom having an a value of 19 and the second having an a value of 18.
Overall, the z, n, and a values can vary for different pairs of atoms depending on their atomic structure and composition.
To know more about N values visit:
https://brainly.com/question/4333050
#SPJ11
is selenide ion a cation or an anion?
Answers
Selenide ion is an anion because it has a negative charge. An ion is an atom or a molecule that has lost or gained one or more electrons, resulting in a net electrical charge. When an ion has a positive charge, it is called a cation, and when it has a negative charge, it is called an anion.
The terms "cation" and "anion" were coined in the late 19th century by the Swedish chemist Svante Arrhenius, who proposed the theory of electrolytic dissociation. According to this theory, when a substance dissolves in water, it dissociates into ions that carry electrical charges. Arrhenius used the terms "cation" and "anion" to describe the positively and negatively charged ions, respectively.
The word "cation" comes from the Greek word "kata," meaning "down," and "ion," meaning "going." The term "anion" comes from the Greek word "ana," meaning "up," and "ion," meaning "going." These terms were chosen because, in an electrolytic solution, cations move towards the cathode (a negatively charged electrode) and anions move towards the anode (a positively charged electrode).
In summary, selenide ion is an anion because it has a negative charge. The terms "cation" and "anion" were coined by Svante Arrhenius to describe the positively and negatively charged ions, respectively, that result from the electrolytic dissociation of substances in solution.
Selenide ion is an anion because it has a negative charge. An ion is an atom or a molecule that has lost or gained one or more electrons, resulting in a net electrical charge. When an ion has a positive charge, it is called a cation, and when it has a negative charge, it is called an anion.
The terms "cation" and "anion" were coined in the late 19th century by the Swedish chemist Svante Arrhenius, who proposed the theory of electrolytic dissociation. According to this theory, when a substance dissolves in water, it dissociates into ions that carry electrical charges. Arrhenius used the terms "cation" and "anion" to describe the positively and negatively charged ions, respectively.
In summary, selenide ion is an anion because it has a negative charge. The terms "cation" and "anion" were coined by Svante Arrhenius to describe the positively and negatively charged ions, respectively, that result from the electrolytic dissociation of substances in solution.
Learn more about selenide ion on:
https://brainly.com/question/29748982
#SPJ6
The first P-wave of an earthquake took 11 minutes to travel to a seismic station from the epicenter of the earthquake. What is the seismic station's distance to the epicenter of the earthquake and how long did it take for the first S-wave to travel that distance?
Distance to epicenter: 3350 km/ S-wave travel time: 4 min 50 sec
Distance to epicenter: 3350 km/ S-wave travel time: 6 min 10 sec
Distance to epicenter: 7600 km/ S-wave travel time: 9 min
Distance to epicenter: 7600 km/ S-wave travel time: 20 min
Answers
Answer: distance to epicenter: 7600 km, s-wave travel time: 20 minutes
How is raw steel made and how is it different from raw iron?
Answers
what product is formed when 2,3,3-trimehtylhept-1-en-6-ynr is reacted with pd/c and h2
Answers
The product formed when 2,3,3-trimethylhept-1-en-6-ynr is reacted with Pd/C and H2 is 2,3,3-trimethylhexane. Let's learn more about the reaction of 2,3,3-trimethylhept-1-en-6-ynr with Pd/C and H2. 2,3,3-Trimethylhept-1-en-6-ynr reacts with hydrogen in the presence of the palladium/carbon catalyst to create 2,3,3-trimethylhexane as the main answer. In this case, palladium on carbon is used as a catalyst to catalyze the hydrogenation reaction.
Palladium (Pd) is an effective hydrogenation catalyst because of its unique electronic and structural properties. Palladium is added to carbon so that it can act as a support system for the palladium metal.
Hydrogenation reactions can be classified as one of two types: catalytic hydrogenation or non-catalytic hydrogenation. In this case, the reaction is catalytic hydrogenation because it occurs in the presence of a catalyst (palladium on carbon). The following is the chemical equation for the reaction: 2,3,3-Trimethylhept-1-en-6-ynr + H2 → 2,3,3-TrimethylhexaneThe reaction takes place in a hydrogenation apparatus under pressure, which is controlled by a pressure regulator. The temperature is kept constant using a water bath or oil bath.
TO know more about that trimethylhept visit:
https://brainly.com/question/12978585
#SPJ11
why does the substitution of ortho or para nitro groups onto chlorobenzene greatly increase the tendency of the chlorine to be displaced by nucleophiles?
Answers
As an electron-withdrawing group, the nitro group reduces the electron density over the benzene ring. Haloarenes are more reactive to nucleophlic substitution when nitro groups are present in the ortho or para position.
What are nucleophiles ?A reactant known as a nucleophile provides an electron pair in order to create a covalent bond. Typically, a nucleophile has a negative charge or is neutral with one or two donateable electrons. Some examples include H2O, -OMe, or -OtBu. The species with a lot of electrons is a nucleophile overall.
A nucleophile is an atom or molecule that seeks the positive center of a chemical process, such as the atom's nucleus, since it has an accessible electron pair for bonding.
To know more about nucleophiles you may visit:
brainly.com/question/28325919
#SPJ4
please help 100 points.

Answers
Answer:
Look up the periodic table and it will tell you what is what or you can ask for a hint and if that doesn't help look up "beryllium Atomic number, Mass number, Protons, electrons, and Neutrons." and do that for the rest of them.
Answer:
dw I gotchu
Explanation:

152. A bubble of gas rises from the bottom of a lake 30 m deep. At what depth will the volume be thrice as great as it was originally (atmospheric pressure =0.76 m of mercury; specific gravity of mercury =13.6 ) ? (Chapter 10) A. 2.42 m B. 4.22 m C. 1.31 m D. 3.11 m
Answers
The correct answer is option B: 4.22 m.
To solve this problem, we can apply Boyle's law and the concept of hydrostatic pressure.
Boyle's law states that the volume of a gas is inversely proportional to its pressure when the temperature remains constant.
Let's assume that the initial volume of the bubble is V and the depth at which the volume becomes thrice as great is H.
We know that the atmospheric pressure is 0.76 m of mercury, and the specific gravity of mercury is 13.6.
At a depth of H, the pressure acting on the bubble is the sum of the atmospheric pressure and the pressure due to the column of liquid above it.
Using the hydrostatic pressure formula:
P = P0 + ρgh
where
P is the pressure at a given depth,
P0 is the atmospheric pressure,
ρ is the density of the liquid,
g is the acceleration due to gravity, and
h is the depth.
In this case, we can use the specific gravity of mercury to find its density:
ρ = (density of water) * (specific gravity of mercury)
Let's calculate the depth at which the volume becomes thrice as great:
First, find the density of mercury:
ρ = (density of water) * (specific gravity of mercury)
= 1000 kg/m³ * 13.6
= 13600 kg/m³
Using the hydrostatic pressure formula:
P1 = P0 + ρgh1
P2 = P0 + ρgh2
Since the volume is inversely proportional to the pressure, we can write:
V2 = (P1 / P2) * V1
3V1 = (P1 / P2) * V1
Simplifying:
3 = (P1 / P2)
Substituting the values:
3 = (P0 + ρgh1) / (P0 + ρgh2)
To find the depth H, we rearrange the equation:
h2 = (P0 + ρgh1) / (ρg) - h1
Substituting the given values:
h2 = (0.76 + (13600 kg/m³ * 9.8 m/s² * 30 m)) / (13600 kg/m³ * 9.8 m/s²) - 0
Calculating the value:
h2 ≈ 4.22 m
Therefore, at a depth of approximately 4.22 m, the volume of the bubble will be thrice as great as it was originally.
The correct answer is option B: 4.22 m.
Learn more about Boyle's law from this link:
https://brainly.com/question/1696010
#SPJ11
in order for a titration to be effective, all of the following must be true of the reaction, except a. reaction must be stoichiometric b. reaction must produce a precipitate c. reaction must be quantitative d. reaction must be rapid
Answers
In order for a titration to be effective, the reaction must produce a precipitate. The correct answer is option B, "reaction must produce a precipitate."
For a titration to be effective, the reaction must be stoichiometric, quantitative, and rapid. A stoichiometric reaction is one in which the amount of reactants is proportional to the amount of products.
A quantitative reaction is one in which all the reactants are consumed, leaving no excess. A rapid reaction is one that occurs quickly and does not take a long time to complete.
However, a reaction producing a precipitate is not necessary for the titration to be effective. Hence option B is correct.
Learn more about titration here:
brainly.com/question/2728613
#SPJ11
what quantity in moles of iron atoms do you have if you have 2.50 × 10²³ atoms of iron. (the mass of one mole of iron is 55.85 g.) quizlet
Answers
There are 4.48 x 10² moles of iron atoms present in 2.50 x 10²³ atoms.
In order to answer this question, we must first determine how many moles of iron atoms are present in 2.50 x 10²³ atoms. To do this, we need the atomic mass of iron which is 55.85 g. This means that one mole of iron has a mass of 55.85 g.
Therefore, in order to determine the number of moles present in 2.50 x 10²³ atoms, we must divide the mass of 2.50 x 10²³ atoms by 55.85 g, which gives us a result of 4.48 x 10² moles of iron atoms. So, the answer to the question is that there are 4.48 x 10² moles of iron atoms present in 2.50 x 10²³ atoms.
know more about iron atoms here
https://brainly.com/question/12898212#
#SPJ11
How does a chemist know that a reaction is an oxidation reduction reaction?.
Answers
A chemist can identify an oxidation-reduction reaction by analyzing whether or not there has been a change in the oxidation number of the reactants. This is done by examining how the reaction affects the movement of electrons between the different molecules involved.
Oxidation is the process by which a molecule loses electrons, while reduction is the process by which a molecule gains electrons. An oxidation-reduction reaction is a reaction that involves the transfer of electrons from one molecule to another. This can be identified by analyzing the change in the oxidation numbers of the different molecules involved.
For example, in the reaction between hydrogen and chlorine, H₂ + Cl₂ → 2HCl, hydrogen is oxidized from an oxidation state of 0 to +1, while chlorine is reduced from an oxidation state of 0 to -1. This indicates that this is an oxidation-reduction reaction.
Learn more about oxidation-reduction reaction here: https://brainly.com/question/4222605
#SPJ11
If an airplane has parts that function like
a person's parts, then what part of the body does the pilot represent?
Explain your reasoning.
Answers
Answer:
Obtaining a Medical Certificate. Most pilots must have a valid medical certificate to exercise the privileges of their airman certificates. Glider and free.
Explanation:
Aviation safety relies heavily on maintenance. When it is not done correctly, it contributes to a significant proportion of aviation accidents and incidents. Some examples of maintenance errors are parts installed incorrectly, missing parts, and necessary checks not being performed.
Hi, help with these two questions.
1. Write the isotope notation for an atom with 9 protons and an atomic mass of 20.
2. Write the isotope notation for an atom with 13 protons and 12 neutrons.
Answers
Answer:
Answered below
Explanation:
1) Atomic number is usually the number of protons
Since the atom is a neutral atom, Number of protons is equal to the number of electrons.
Thus means that we have 9 electrons and our atomic number is 9.
Atomic mass number is given as 20.
The is topic notation makes use of the atomic number and mass number.
Atomic number of 9 denotes fluorine.
Thus, the isotopic notation is written as attached;
2) we are told that an atom has 13 protons and 12 neutrons.
Similar to A above,
Atomic number is 13.
Now, number of neutrons + number of protons = mass number.
Thus;
Mass number = 13 + 12 = 25
Element with atomic number of 12 is magnesium
Thus,isotopic notation is as attached.

Which of the following correctly predicts the most likely mode of radioactive decay for the nuclide As3384As3384?
Answers
Of the following correctly predicts the most likely mode of radioactive decay for the nuclide \(As^{33}_{84}\)
The nuclide \(As^{33}_{84}\) has an atomic number of 33, indicating that it is arsenic. To predict the most likely mode of radioactive decay for \(As^{33}_{84}\), we need to consider its position on the periodic table and the stability of its nucleus
\(As^{33}_{84}\) falls into the category of a stable nuclide since it has a stable atomic number. Stable nuclides do not undergo radioactive decay. Therefore, it is unlikely that \(As^{33}_{84}\) would undergo spontaneous radioactive decay through alpha decay (emitting an alpha particle), beta decay (emitting a beta particle), or gamma decay (emitting gamma radiation). Nuclides that are unstable and undergo radioactive decay typically have atomic numbers higher than the stable region of the periodic table or have an imbalance of protons and neutrons in the nucleus. However, as \(As^{33}_{84}\) is a stable nuclide, it is not expected to undergo any form of radioactive decay. Hence, the most likely mode of radioactive decay for the nuclie \(As^{33}_{84}\) is no decay at all since it is a stable nuclide.
Learn more about nuclide here:
https://brainly.com/question/32387415
#SPJ11
What are the consequences of purposely having an excess of one of the reactants?
Answers
Answer:
When one reactant is in excess, there will always be some left over. The other reactant becomes a limiting factor and controls how much of each product is produced. While using excess reactants can help to increase percentage yields, this is at the expense of atom economy.
Explanation:
For the vaporization of bromine, Br2(l)->Br2(g), at what kelvin temperature will the process be spontaneous?
Answers
The vaporization of bromine will be spontaneous at temperature 298.15 K (25°C).
In order to determine the Kelvin temperature at which the vaporization of bromine, Br₂(l) → Br₂(g), will be spontaneous, we need to consider the change in Gibbs free energy (ΔG) for the process.
If ΔG is negative, the process is spontaneous at that temperature, indicating that the vaporization of bromine will occur without any external intervention. On the other hand, if ΔG is positive, the process is non-spontaneous at that temperature and will not occur without the input of energy.
The equation for ΔG is given by;
ΔG = ΔH - TΔS
where ΔH is the enthalpy change and ΔS is the entropy change for the vaporization process, and T is the temperature in Kelvin.
The values for ΔH and ΔS for the vaporization of bromine at 25°C (298.15 K) are as follows;
ΔH = 31.4 kJ/mol
ΔS = 93.1 J/(mol·K)
Substituting these values into equation ΔG;
ΔG = 31.4 kJ/mol - 298.15 K × (93.1 J/(mol·K)/1000 J/kJ) = 31.4 kJ/mol - 0.0277 kJ/mol
ΔG = 31.4 kJ/mol - 0.0277 kJ/mol = 31.3723 kJ/mol
Since ΔG is negative, the vaporization of bromine will be spontaneous at any temperature above 298.15 K (25°C).
To know more about vaporization here
https://brainly.com/question/8605699
#SPJ1
Help with this question!!!

Answers
Answer:
diple doble
Explanation:
a change in pressure affects the chemical equilibrium of the reaction system under what condition?
Answers
Answer:
A change in pressure will affect the equilibrium position of the reaction if one or more of the reactants or products are in the gaseous phase.
Explanation:
Le Chapelries principle states that whenever a change is brought about to a system under chemical equilibrium, the equilibrium shifts in a manner to reverse this change. So if the pressure of a reaction mixture is increased, the equilibrium will shift towards the side with the lower number of molecules, and if the pressure is reduced, the equilibrium will shift to the side with the greater number of molecules.
Hi :)
The answer is
The equilibrium will shift to minimise that change.
If the pressure is decreased the equilibrium will shift to favour an increase in pressure.
Hope this helps~
~NatLikesAnime~
#LearningWithBrainly
which statement regarding the chemical grooming of pyruvate is false? which statement regarding the chemical grooming of pyruvate is false? each pyruvate molecule has a co2 added and then joins with an nadh
Answers
The statement that is false regarding the chemical grooming of pyruvate is "each pyruvate molecule has a CO2 added and then joins with an NADH.
Pyruvate is the end product of glycolysis that further undergoes chemical grooming in the presence of oxygen to produce ATP. The complete oxidation of glucose produces a total of 36-38 ATPs per molecule.
Pyruvate is oxidized to produce Acetyl-CoA. During this process, the carboxyl group of pyruvate is removed and given off as CO2. This is known as decarboxylation.
The remaining 2-carbon molecule is then oxidized by the removal of electrons by the NAD+ which is reduced to NADH. This is called oxidative decarboxylation, and its purpose is to prepare the substrate for energy production.
The correct statement regarding the chemical grooming of pyruvate is, "Each pyruvate molecule loses a CO2 molecule and then joins with a coenzyme A to form acetyl-CoA, producing an NADH molecule."
Learn more about the chemical:
https://brainly.com/question/11231920
#SPJ11
Rank the layers of the atmosphere based on the energy of the photons that are typically emitted there, from highest to lowest.
Answers
The layers of the atmosphere can be ranked based on the energy of the photons that are typically emitted there, from highest to lowest as Thermosphere,Mesosphere,Stratosphere and Troposphere respectively.
Based on the energy of the photons typically emitted, the layers of the atmosphere can be ranked as follows:
1. Thermosphere: This layer has the highest energy of photons as it is the region where the sun's radiation is absorbed and ionizes the gas particles. This ionization process releases high-energy photons, including ultraviolet and X-rays.
2. Mesosphere: This layer has a lower energy of photons than the thermosphere. It is the region where meteoroids burn up upon entering the Earth's atmosphere, releasing photons in the form of light.
3. Stratosphere: This layer has a lower energy of photons than the mesosphere. It is the region where ozone is present, which absorbs high-energy ultraviolet radiation from the sun and emits lower energy photons in the form of heat.
4. Troposphere: This layer has the lowest energy of photons as it is the region closest to the Earth's surface and is primarily heated by convection from the ground. The photons emitted here are primarily in the form of infrared radiation.
Learn more about photons here:https://brainly.com/question/29254702
#SPJ11
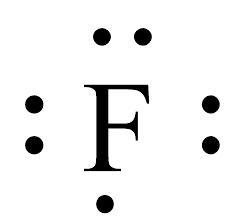
Draw the correct Lewis dot structure from the given shorthand notation below: PLS HELP

Answers
The Lewis structure of the element have been shown in the image attached.
Lewis dot structure of an element:The valence electrons of an atom or molecule are depicted in a simplified manner by the Lewis structure, commonly referred to as the Lewis dot structure or electron dot structure. Gilbert N. Lewis, an American scientist, created it.
The valence electrons of an atom are shown in a Lewis structure as dots surrounding the element's symbol. These dots' placement reveals details about the connectivity and atom-atom bonding in a molecule.
Learn more about Lewis structure:https://brainly.com/question/29756546
#SPJ1
I need help writing about transformation of energy but it has to have a title,objective,hypothesis, procedures and data analysis and conclusion
Answers
Answer:
Title: Investigating the Transformation of Energy
Objective: The objective of this experiment is to observe and analyze the transformation of energy between different forms, such as kinetic, potential, thermal, and electrical energy.
Hypothesis: It is hypothesized that energy can be transformed from one form to another, but the total amount of energy in a closed system remains constant (the law of conservation of energy).
Procedures:
Gather materials, including a pendulum, stopwatch, ruler, and potential energy toy.Set up the pendulum and measure its length, mass, and amplitude.Start the pendulum and measure its period and velocity.Use the potential energy toy to measure the potential energy stored in the toy.Use the stopwatch to measure the time it takes for the potential energy toy to hit the ground.Use a thermometer to measure the temperature of water before and after heating it with an electric heater.Measure the voltage and current of the electric heater and calculate the power used.Measure the distance traveled and time taken by a toy car moving down a ramp, and calculate its average speed.Use a multimeter to measure the voltage and current of a battery, and calculate its electrical energy.Data Analysis:
Analyze the data collected from the pendulum experiment to calculate its kinetic and potential energy, and compare the total energy to the initial potential energy of the pendulum.Calculate the gravitational potential energy of the potential energy toy and compare it to the kinetic energy just before it hits the ground.Use the data collected from the electric heater experiment to calculate the energy used to heat the water and compare it to the electrical energy used by the heater.Calculate the kinetic energy of the toy car and compare it to the gravitational potential energy of the ramp.Calculate the electrical energy stored in the battery and compare it to the energy used to power a light bulb.Conclusion:
The results of this experiment support the hypothesis that energy can be transformed from one form to another, but the total amount of energy in a closed system remains constant. This experiment demonstrated the transformation of energy between kinetic, potential, thermal, and electrical forms, and showed how energy can be transferred and used to do work. Understanding the transformation of energy is important in many fields, including physics, engineering, and environmental science.
which element in the synthesis reaction for rusting is being oxidized and which is being reduced? how do you know?
Answers
In the synthesis reaction for rusting, iron (Fe) is oxidized and oxygen (O₂) is being reduced. We know this because during the rusting process, iron loses electrons and forms iron oxide (Fe₂O₃), which indicates oxidation.
In the synthesis reaction for rusting, iron (Fe) is oxidized and oxygen (O₂) is being reduced. This can be determined by examining the oxidation states of each element before and after the reaction. In the reactants, iron has an oxidation state of 0 and oxygen has an oxidation state of 0. In the products, iron has an oxidation state of +2 and oxygen has an oxidation state of -2.
Since the oxidation state of iron has increased (from 0 to +2), it has undergone oxidation. Since the oxidation state of oxygen has decreased (from 0 to -2), it has undergone reduction. Therefore, iron is the element being oxidized and oxygen is the element being reduced in the synthesis reaction for rusting.
You can learn more about synthesis reactions at: brainly.com/question/2165817
#SPJ11
it is the minimum amount of solute that can be dissolved in the solvent
A. Salt Solution C. Saturated Solution
B. Sugar Solution D.
Answers
Answer:A
Explanation:
the mass of solution is equivalent to the mass of the?
A. solute + solvent
B.solvent
C.solute-solvent
D.solute
Answers
The mass of solution is equivalent to the mass of the solute and solvent.
What is solute?The material whose dissolves is known as a solute, as well as the substance
What is solvent?The solute is dissolved to produce a solution, is known as a solvent.
Solution is made by solvent , solute also. So, by adding mass of solvent and solute mass of a particular solution could be found.
It can be expressed as:
Mass of solution = mass of solute + mass of solvent
Therefore, the mass of solution is equivalent to the mass of the solute and solvent.
To know more about solute and solvent.
https://brainly.com/question/14797683
#SPJ2
given an aqueous solution in which the [h ] = 2.5 x 10-7, what is the molar hydroxide ion concentration?
Answers
The molar hydroxide ion concentration in the given aqueous solution is 4.0 x 10⁻⁸ M.
To find the molar hydroxide ion concentration, we will use the concept of the ion product constant of water (Kw) and the provided hydrogen ion concentration [H+].
The ion product constant of water, Kw, is given by the product of the hydrogen ion concentration [H+] and hydroxide ion concentration [OH-]. In pure water at 25°C, Kw is 1.0 x 10⁻¹⁴. The formula is:
Kw = [H+] × [OH-]
We are given [H+] = 2.5 x 10⁻⁷. We can now find the [OH-] concentration using the formula:
[OH-] = Kw / [H+]
Plug in the values:
[OH-] = (1.0 x 10^-14) / (2.5 x 10⁻⁷)
[OH-] = 4.0 x 10⁻⁸
This calculation is based on the relationship between the ion product constant of water and the concentrations of hydrogen and hydroxide ions.
You can learn more about hydroxide ions at: brainly.com/question/14576028
#SPJ11