Answers
Related Questions
13. Choose the geologic formation that fits the description: the world's longest mountain chain.
Answers
The mid-ocean ridge would be the longest and largest mountainous region. With a circumference of 40,389 miles, it genuinely is a world landmark.
The water covers around 90% of the mid-ocean ridge system. The world is crisscrossed by a system of mountains as well as valleys that resembles the seams in a baseball. Tectonic plate movement on Earth is what creates it.
Mountain ranges like the Rockies had also often grown and fallen over the evolution of our planet. Whenever two continents clash, mountains are created. Due to their identical weight as well as diameter, neither plate would bury beneath the other.
In a process known as plate tectonics, sections of such Earth's mantle, known as plates, collide with one another as well as buckle up like such a rear bumper in a frontal collision to build the world's largest mountain ranges.
To know more about mountain chain
https://brainly.com/question/29276761
#SPJ1
Rearrange the equation to isolate . =
If =8.00 , =9.00 , and =3.00 , what is the value of ?
Answers
Answer:
Explanation:
8.00 + 9.00 + 3.00 = 20 i dont really know what your asking but i bet your really smart!
A blood sample is left on a phlebotomy tray for 4 hours before it is delivered to the laboratory. Which group of tests could be performed:
Answers
Substance whose smell changes in acidic or basic solutions
Answers
Answer:
Olfactory indicators
Explanation:
The substance whose odour changes in an acidic of basic medium are called olfactory indicators. In an olfactory indicator , smell varies depending on whether it is mixed with an acidic or basic solution.
Which statements correctly describe metallic bonds
Answers
Answer:
E. They form because electrons can move freely between atoms.
Explanation:
Metallic bonding is a type of chemical bonding that arises from the electrostatic attractive force between conduction electrons and positively charged metal ions.
Joseph rides his bike for 30 miles in one hour
going west . Is it speed velocity or acceleration
Answers
Answer:
velocity
Explanation:
because he is going 30 miles west
What element has an atomic mass 4?
Answers
Answer:
Helium has atomic mass 4.002602.
So the answer to yr question is helium.
Remember that the solution must be in complete form: reaction equations; calculation formulas with numerical values of physical quantities placed in them., units of measurement!
The students were given the task to obtain 10 g of Cu(OH)2 experimentally. In the laboratory, students had access to a 10% CuSO4 solution and a 20% NaOH solution. Calculate how many ml of CuSO4 solution and how many ml of NaOH solution are needed to complete the given task! (Density of 10% CuSO4 solution ρ = 1.107 g/ml and density of 20% NaOH solution ρ = 1.219 g/ml)
Answers
We need 147.5 ml of 10% CuSO4 solution and 33.6 ml of 20% NaOH solution to obtain 10 g of Cu(OH)2.
To obtain 10 g of Cu(OH)2, we need to start by writing the balanced chemical equation for the reaction between copper sulphate (CuSO4) and sodium hydroxide (NaOH):
CuSO4 + 2NaOH -> Cu(OH)2 + Na2SO4
From the balanced equation, we can see that one mole of CuSO4 reacts with two moles of NaOH to produce one mole of Cu(OH)2 and one mole of Na2SO4.
The molar mass of Cu(OH)2 is 97.56 g/mol. Therefore, we need 10 g / 97.56 g/mol = 0.1024 mol of Cu(OH)2.
To determine how much CuSO4 solution and NaOH solution we need, we can use the following equations:
Amount of CuSO4 required = 0.1024 mol x (159.61 g/mol) / 0.1 = 163.38 g
Amount of NaOH required = 0.2048 mol x (40.00 g/mol) / 0.2 = 40.96 g
Where the first equation is using the molar mass of CuSO4 and the density of the solution to determine the required volume. The same idea is used in the second equation but with NaOH.
Using the density of the solutions, we can convert the required mass to volume:
Volume of CuSO4 solution = 163.38 g / 1.107 g/ml = 147.5 ml
Volume of NaOH solution = 40.96 g / 1.219 g/ml = 33.6 ml
Therefore, we need 147.5 ml of 10% CuSO4 solution and 33.6 ml of 20% NaOH solution to obtain 10 g of Cu(OH)2.
For such more questions on solution
https://brainly.com/question/25326161
#SPJ11
Why do we use commas after transition words?
Commas are extra punctuation.
Commas show readers when something should be shouted.
Commas indicate a natural pause in the sentence.
Commas make the sentence look nicer.
Answers
Answer:
C - commas indicate a natural pause in the sentence
Use the standard reduction potentials located in the 'Tables' linked above to calculate the equilibrium constant for the reaction:
2H+(aq) + Cu(s) H2(g) + Cu2+(aq)
Hint: Carry at least 5 significant figures during intermediate calculations to avoid round off error when taking the antilogarithm. You may use the OWL references to find the values you may need in this question.
Answers
Answer:
3.3 * 10^-12
Explanation:
The balanced equation of the reaction is;
2H+(aq) + Cu(s) ---------> H2(g) + Cu2+(aq)
Hence two electrons were transferred so n=2
E°cell = E°cathode - E°anode
E°cell = 0 V - 0.34 V
E°cell = - 0.34 V
Then;
E°cell = 0.0592/n log K
Substituting values;
- 0.34 = 0.0592/2 log K
- 0.34/0.0296 = log K
-11.486 = log K
K = Antilog (-11.486)
K = 3.3 * 10^-12
Ammonium sulfate (NH4)2SO4 is a common substance used to treat highly alkaline soils. If a particular piece of land requires you to apply 17.2 g of ammonium sulfate how many mL of a 6.50 M ammonium sulfate solution do you need to apply to that area of land?
Answers
Answer:
0.02 L (20 mL) of ammonium sulfate solution.
Explanation:
Solutions => Molarity.
You can note that we have a given mass of ammonium sulfate ((NH4)2SO4) and its concentration (molar), so to solve this problem we have to understand first what is the molarity: the molarity (M) of a solution is the number of moles of solute dissolved in one liter of solution. The formula of molarity is the following:
\(Molarity(M)=\frac{mole\text{s of solute}}{liter\text{s of solution}}=\frac{mol}{L}.\)We can solve for 'liters of solution' but we don't know what is the value of moles of solute because we have the amount of solute in grams (mass).
We can find the number of moles of ammonium sulfate by using its molar mass, which is 132 g/mol. Remember that you can calculate the molar mass of a compound using the periodic table. The conversion from grams to moles will look like this:
\(17.2\text{ g \lparen NH}_4)_2SO_4\cdot\frac{1\text{ mol \lparen NH}_4)_2SO_4}{132\text{ g \lparen NH}_4)_2SO_4}=0.130\text{ moles \lparen NH}_4)_2SO_4.\)We have 0.130 moles of ammonium sulfate in 17.2 g of ammonium sulfate, so now we can replace the data that we have if we solve for 'liters of solution', like this:
\(\begin{gathered} liter\text{s of solution=}\frac{moles\text{ of solute}}{molarity}\text{,} \\ \\ liter\text{s of solution=}\frac{0.130\text{ moles}}{6.50\text{ M}}\text{,} \\ \\ liters\text{ of solution=0.02 L.} \end{gathered}\)Remember that 1 L equals 1000 mL, so the conversion from 0.02 L to mL is:
\(0.02\text{ L}\cdot\frac{1000\text{ mL}}{1\text{ L}}=20\text{ mL.}\)The answer would be that we need 0.02 L (20 mL) of ammonium sulfate solution to apply to the piece of land.
100 points help me asap!!!

Answers
When the acidic byproduct of plaque tears away at the teeth's enamel, demineralization of the teeth takes place.
Thus, Mineral molecules like calcium and phosphate give enamel, which is made of minerals, a lot of its strength and hardness.
When a tooth experiences tooth decay, the minerals inside it start to wear away (thus the term "demineralization") and render the tooth's enamel permeable, which can occasionally result in cavities or other dental problems.
Although the definition of bone demineralization may make you nervous, it actually happens naturally throughout the course of a tooth's lifespan.
Thus, When the acidic byproduct of plaque tears away at the teeth's enamel, demineralization of the teeth takes place.
Learn more about demineralization, refer to the link:
https://brainly.com/question/17794394
#SPJ1
A solution of the ionic saltNH4Cl would havepH.ABan acidica basica neutral

Answers
ANSWER
Acidic
EXPLANATION
Ammonium chloride is an acidic salt prepared the combination of a strong acid and a weak base
The strong acid is the hydrochloric acid and the weak base is the ammonium hydroxide.
Below is the reaction between hydrochloric acid and ammonium hydroxide
\(\text{ HCl}_{(aq)}\text{ + NH}_4OH_{(aq)}\rightarrow\text{ NH}_4Cl\text{ + H}_2O\)Therefore, the pH of NH4Cl in solution is acidic.
The density of an object never changes when the temperature of that object changes
true or false?
Answers
What is radiative equilibrium?
Answers
Answer:
Radiative equilibrium is the condition where the total thermal radiation leaving an object is equal to the total thermal radiation entering it. It is one of the several requirements for thermodynamic equilibrium, but it can occur in the absence of thermodynamic equilibrium.
Answer:
Radiative equilibrium is the condition where the total thermal radiation leaving an object is equal to the total thermal radiation entering it. It is one of the several requirements for thermodynamic equilibrium, but it can occur in the absence of thermodynamic equilibrium.
Hope this helps :)
what are 4 molecules made of the same substance
Answers
Answer:
H2O (water)N2 (nitrogen)O3 (ozone)CaO (calcium oxide)Explanation:HOPE IT HELPS
a solution containing a kcl dissolved in water will have a than that of pure water. multiple choice higher boiling point and a lower freezing point none of the answers can be determined with the information provided. lower boiling point and a lower freezing point lower boiling point and a higher freezing point higher boiling point and a higher freezing point
Answers
A solution containing a KCl dissolved in water will have a higher boiling point and a lower freezing point than that of pure water.
Elevation in boiling point is a phenomenon that describe the boiling point of a liquid will be higher when another compound is added. Which means a solution has higher boiling point than pure solvent .
Depression in freezing point is a phenomenon that describe the freezing point of a liquid will be lower when another compound is added. Which means a solution has lower freezing point than pure solvent.
Thus, The boiling point and the freezing point of a solution of KCl in water will be greater and lower, respectively, than that of pure water.
To learn more about Boiling & Freezing point, Here :
https://brainly.com/question/7197568?referrer=searchResults
#SPJ4
3. What form of energy causes water in the ocean to evaporate? chemical thermal mechanical potential
Answers
Answer:
i think the answer is b but dont knock me if its not
Explanation:
pls help I’ll give allot of points I need all the answers pls anybody help!!!
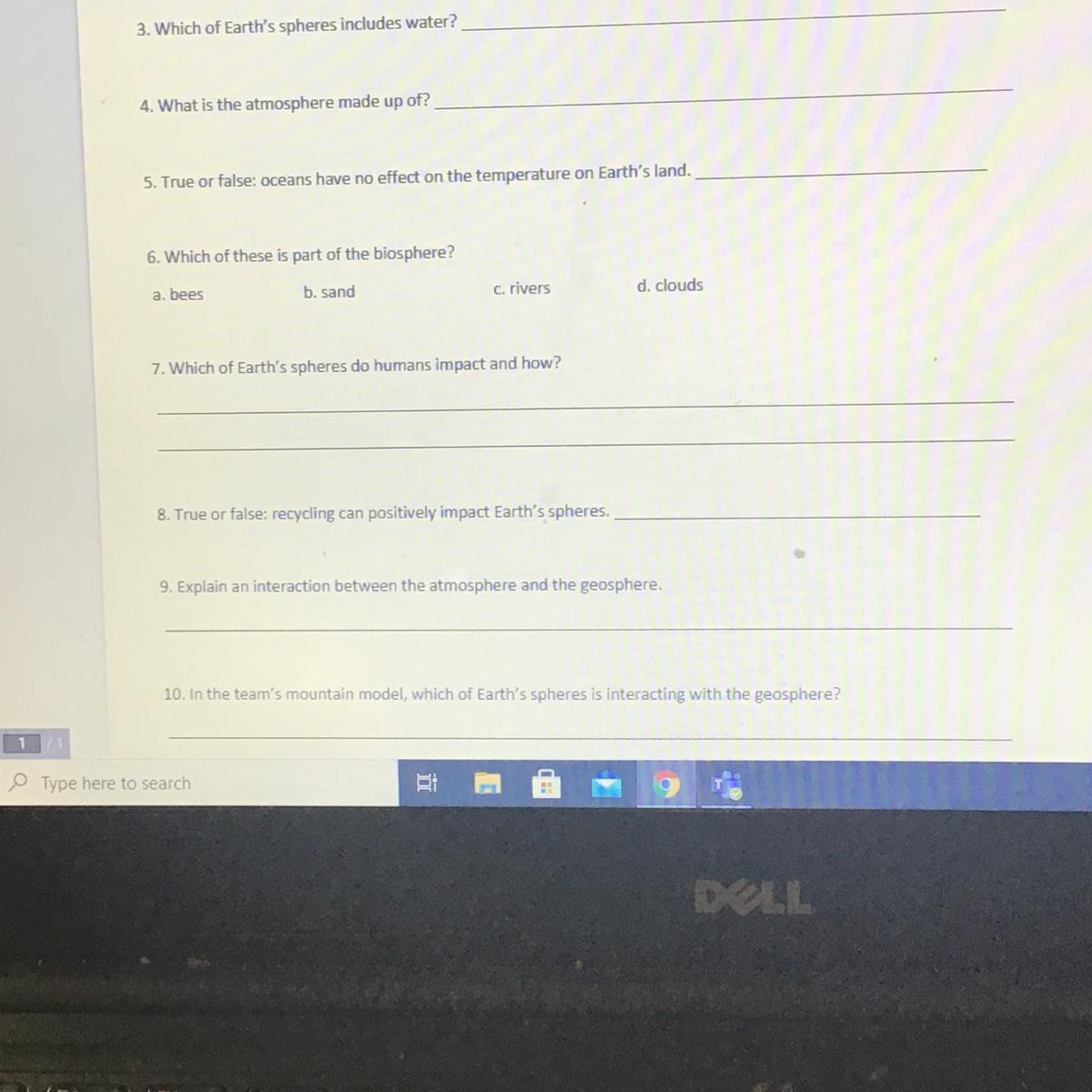
Answers
4: Gases
5: False - I think
6: D - Clouds
In the reaction 2 RbNO3 → 2 RbNO2 + O2 , how many liters of oxygen are producedwhen 5.0 moles of rubidium nitrate decompose?
Answers
According to the explanation given in our previous session, now we have a similar question but with a slight difference, but first let's set up the reaction:
2 RbNO3 -> 2 RbNO2 + O2
We have 5.0 moles of RbNO3
From the molar ratio we see that 2 moles of RbNO3 is equal to 1 mol of O2, therefore if we have 5 moles of RbNO3, we will have 2.5 moles of O2 being produced.
Since the question is asking "how many liters" we have to assume that we are dealing with gases and these gases are at STP (standard temperature and pressure), which is T = 273K and P = 1 atm, in these specfici conditions, 1 mol of gases will have a volume of 22.4 Liters, therefore if O2 has 2.5 moles of O2
1 mol = 22.4L
2.5 moles = x L
x = 56 Liters of O2 are being produced with 5.0 moles of RbNO3
What is the molecular formula of each of the following
compounds?
(a) empirical formula CH₂, molar mass = 84 g/mol
(b) empirical formula NH₂Cl, molar mass = 51.5 g/mol
Answers
(a) the molecular formula of the compound is C₆H₁₂.
(b) the molecular formula of the compound is NH₂Cl.
(a) Given the empirical formula CH₂ and a molar mass of 84 g/mol, we need to determine the molecular formula. To do so, we need to find the factor by which the empirical formula needs to be multiplied to achieve the given molar mass.
The empirical formula CH₂ has a molar mass of 14 g/mol (12 g/mol for carbon + 2 g/mol for hydrogen).
To find the factor, we divide the molar mass by the empirical formula mass:
Factor = (molar mass) / (empirical formula mass) = 84 g/mol / 14 g/mol = 6
Therefore, the molecular formula is obtained by multiplying the empirical formula by the factor:
CH₂ × 6 = C₆H₁₂
Thus, the molecular formula of the compound is C₆H₁₂.
(b) Given the empirical formula NH₂Cl and a molar mass of 51.5 g/mol, we follow a similar approach.
The empirical formula NH₂Cl has a molar mass of 51.5 g/mol (14 g/mol for nitrogen + 2 g/mol for each hydrogen + 35.5 g/mol for chlorine).
To find the factor, we divide the molar mass by the empirical formula mass:
Factor = (molar mass) / (empirical formula mass) = 51.5 g/mol / 51.5 g/mol = 1
Therefore, the molecular formula is the same as the empirical formula: NH₂Cl
Hence, the molecular formula of the compound is NH₂Cl.
for more questions on molecular
https://brainly.com/question/24191825
#SPJ8
Please help fast
All four referenced Greek thinkers: Democritus, Aristotle, Archimedes, and Anaxagoras, observed Nature and argued for his theory of
the composition of matter and natural laws. Only one of them tested his hypothesis and proposed a natural laws based on reproducible
observations, controlled experiments, and mathematical reasoning. All others used logic and thought experiments, as philosophers do,
to support their theories. Who is the experimental scientist in this group?
O Democritus
O Aristotle
O Archimedes
O Anaxagoras
Answers
Answer:
Anaxagoras was perhaps the first literate person to attempt to explain physical phenomena rationally, basing his ideas upon careful observations and simple experiments. This is fundamental to modern science and is the sine qua non of environmental study.
An error during which cellular process would create a gene mutation?
Answers
An error during DNA replication would create a gene mutation.
During DNA replication, the genetic information in a cell is copied to make new DNA molecules. However, mistakes can occur during this process, leading to changes in the DNA sequence, which can result in a mutation. Mutations can also be caused by exposure to environmental factors, such as radiation or chemicals, which can damage the DNA molecule directly or affect the cellular processes involved in DNA replication.
Mutations can have a variety of effects on the organism, ranging from no effect to causing serious health problems or even death. Gene mutations can also be inherited from a parent, which can result in genetic disorders or predisposition to certain diseases. Therefore, it is important to understand the mechanisms of gene mutations and their potential impacts on organisms.
To know more about the Gene mutation, here
https://brainly.com/question/15448555
#SPJ1
What is the name of Bel on the periodic table
Answers
Answer:
Nobelium or Beryllium
help answer this 50 points for each page!!!!


Answers
Thanks for the points, I need them to help other people,
Thanks,
Tqkeoi.
What does cellular respiration do?
Break down sugar and release energy for an
organism to use
Create sugar filled with energy
Deter predators
Form glucose from hydrogen, oxygen, and carbon
:D
help asap
Answers
If one force on an object is 5 N upward and the other is 10 N downward what is the objects motion?
Answers
To solve this we must be knowing each and every concept related to force. Therefore, the objects motion will be towards downward with a force of 5 N.
What is force?A force is an outside entity that has the power to alter a body's state of rest or motion. It has a direction and a magnitude. The point where forces are applied is determined by the the force's direction as well as the application of the force.
Newton and dyne units are used to measure the force exerted on an item. When using the centimeter kilogram second system of units, force is measured in dynes (CGS unit). In the common international system of units, it is denoted by the letter Newton (N) (SI unit).
upward force=5 N
downward force= 10 N
total force acting on body=downward force-upward force
= 10 N - 5 N
=5 N downward force
The objects motion will be towards downward with a force of 5 N.
Therefore, the objects motion will be towards downward with a force of 5 N.
To learn more about force, here:
https://brainly.com/question/13014979
#SPJ5
El butano, C4H10, se quema en presencia de oxígeno gas, O2, y se produce dióxido de carbono, CO2, y agua. ¿Cuántos kg de CO2 se obtendrán al quemarse 12 kg de butano?
Answers
Answer:
don't know really and don't know at alll
Question 10
(05.06 HC)
A 3.81-gram sample of NaHCO3 was completely decomposed in an experiment.
2NaHCO3-Na₂CO3 + H₂CO3
In this experiment, carbon dioxide and water vapors combine to form H₂CO3. After decomposition, the Na₂CO3 had
a mass of 2.86 grams.
1. Determine the mass of the H₂CO3 produced.
2. Calculate the percentage yield of H₂CO3 for the reaction. Show your work or describe the calculation
process in detail,
(10 points)
Answers
Answer:
1. the mass of H2CO3 produced is 1.675 grams.
2. the percentage yield of H2CO3 is 59.7%.
Explanation:
1. Using stoichiometry, we can find the moles of NaHCO3 and Na2CO3 produced in the reaction:
2 NaHCO3 -> Na2CO3 + H2CO3
Moles of NaHCO3 = 3.81 g / 84.01 g/mol = 0.04532 mol
Moles of Na2CO3 = 2.86 g / 105.99 g/mol = 0.02700 mol
Since the reaction produces an equal amount of Na2CO3 and H2CO3, we know that 0.02700 mol of H2CO3 was produced.
To find the mass of H2CO3 produced, we can use its molar mass:
Mass of H2CO3 = 0.02700 mol x 62.03 g/mol = 1.675 g
Therefore, the mass of H2CO3 produced is 1.675 grams.
2. The theoretical yield of H2CO3 is the amount that would be produced if the reaction went to completion and all of the NaHCO3 was converted to Na2CO3 and H2CO3. We can calculate the theoretical yield of H2CO3 by multiplying the moles of NaHCO3 used in the reaction by the molar mass of H2CO3:
Theoretical yield of H2CO3 = 0.04532 mol x 62.03 g/mol = 2.806 g
The percentage yield is calculated by dividing the actual yield (1.675 g) by the theoretical yield (2.806 g) and multiplying by 100:
Percentage yield of H2CO3 = (1.675 g / 2.806 g) x 100% = 59.7%
Therefore, the percentage yield of H2CO3 is 59.7%.
List the 2 pKa's for H2SO4