what is the density in g/l of co at 1140 torr and 75.0 °c?
Answers
The density of CO gas at 1140 torr and 75.0 °C is 14.8 g/L.
To solve for density (d), we can use the ideal gas law equation, which states that PV = nRT, where P is the pressure, V is the volume, n is the number of moles, R is the gas constant, and T is the temperature.
First, we need to convert the pressure from torr to atm, which is 1140/760 = 1.50 atm.
Next, we need to convert the temperature from Celsius to Kelvin, which is 75.0 + 273.15 = 348.15 K.
We can assume that we have one mole of CO gas, since the ideal gas law equation uses moles. Therefore, n = 1.
The gas constant R is 0.0821 L·atm/(mol·K).
Now we can rearrange the ideal gas law equation to solve for V:
V = nRT/P
V = (1 mol)(0.0821 L·atm/(mol·K))(348.15 K)/(1.50 atm)
V = 1.89 L
Finally, we can calculate density using the formula:
d = mass/volume
Since we have one mole of CO gas, we can use its molar mass of 28.01 g/mol.
mass = n × molar mass
mass = (1 mol)(28.01 g/mol)
mass = 28.01 g
d = mass/volume
d = 28.01 g/1.89 L
d = 14.8 g/L
Therefore, the density of CO gas at 1140 torr and 75.0 °C is 14.8 g/L.
Know more about density here:
https://brainly.com/question/1354972
#SPJ11
Related Questions
what occurs when the two solids are placed in contact with each other?
Answers
Answer:
I think its heat flow or conduction
Answer:
Heat energy will flow from one to the other.
Explanation:
This is called "heat transfer" or the transfer of heat, when you place two things together the object was a warmer temperature will transfer it's heat into the object with the lower temperature. For example if you put boiling water in a glass bowl the heat from the water will transfer to the bowl increasing it's temperature.
Hope this helps.
There are 4.78 g of dry LICIO4 and
2.43 g H₂O in the sample.
Step 2: Determine the moles of water and anhydrous compound.
Li = 6.94 g/mol, Cl = 35.45 g/mol,
H = 1.01 g/mol, O = 16.00 g/mol
How many moles of LICIO4 are present?
[?] mol LICIO4
Answers
Answer:
The number of moles of H₂O is 0.135 mol.
The number of moles of LiClO₄ is 0.0449 mol.
Explanation:
Mole is an important standard unit used for the measurement of large quantities of atoms, molecules, or other particles. One mole is equal to 6.022×10²³ units.
The number of moles of a substance is calculated by:
\(\frac{mass of substance}{molecular weight of substance}\)
To find the number of moles of H₂O:
Mass of H₂O in the sample = 2.43g
The molecular weight of H₂O = 18.02g
Number of moles = \(\frac{2.43}{18.02}\) = 0.135 mol.
To find the number of moles of LiClO₄:
Mass of LiClO₄ given = 4.78g
The molecular weight of LiClO₄ = 106.39g
Number of moles = \(\frac{4.78}{106.39}\) = 0.0449 mol.
Learn more about Moles here:
https://brainly.com/question/855186
#SPJ2
Answer:
0.0449
Explanation:
No probelm
plants use light, carbon dioxide, and water to create sugar. this sugar can later be converted into atp to power the cell. which property of life does this represent?
Answers
The property of life this represent is photosynthesis.
Photosynthesis is a process in which plants use sunlight, carbon dioxide, and water to produce sugar. This sugar is subsequently converted into ATP, which is used to power the cell. This represents the characteristic of life known as energy processing. The photosynthesis process requires three important ingredients; carbon dioxide (CO2), light, and water (H2O).
When these ingredients are mixed together, the process of photosynthesis begins. In plants, photosynthesis occurs in chloroplasts. These organelles contain chlorophyll, which is a green pigment that absorbs light.The energy absorbed from sunlight is utilized to transform carbon dioxide and water into glucose and oxygen. Oxygen is then released from the plant through tiny pores called stomata. Glucose, on the other hand, is converted to ATP through the process of cellular respiration.
ATP is then used to power various cell functions.The process of photosynthesis is critical to the life of a plant. It allows the plant to produce its own food, which is then used to provide energy for all cellular functions. This represents the characteristic of life known as energy processing.Plants are known as autotrophs because they create their own food. In contrast, animals are heterotrophs because they depend on other organisms for food.
Learn more about photosynthesis -
brainly.com/question/20861367
#SPJ11
differentiate between atoms and their ions on the basis of their stability.
Answers
Answer:
The difference between an atom and an ion has to do with net electrical charge. An ion is a particle or collection of particles with a net positive or negative charge. ... A stable atom contains the same number of electrons as protons and no net charge. When electrons are added or removed, the stable atom becomes an ion.Apr 12, 2015
Explanation:
hope this helps ✌️
How many grams are there in 6.98 x 1025 molecules of H2?
Answers
There are a couple of steps we need to follow to answer this question. First, we need to determine the molar mass of H2, which is 2 grams per mole (since H2 has a molar mass of 2).
Next, we can use Avogadro's number (6.022 x 10^23 molecules per mole) to convert the number of molecules we have to moles.
6.98 x 10^25 molecules / 6.022 x 10^23 molecules per mole = 115.8 moles
Finally, we can use the molar mass to convert the moles to grams:
115.8 moles x 2 grams per mole = 231.6 grams
Therefore, there are 231.6 grams in 6.98 x 10^25 molecules of H2.
Answer more than 100 words: To summarize, we first found the molar mass of H2, which is 2 grams per mole. We then used Avogadro's number to convert the number of molecules to moles and found that we had 115.8 moles of H2. Finally, we multiplied the number of moles by the molar mass to get the number of grams, which was 231.6 grams. This calculation is an example of how to convert between the number of molecules of a substance and its corresponding mass in grams, which can be a useful tool in chemistry and other scientific fields.
There are 233.49 grams in 6.98 x 10²⁵ molecules of H₂. To determine the number of grams in 6.98 x 10²⁵ molecules of H₂, we first need to calculate the molar mass of H₂, which is 2.016 g/mol.
Next, we can use Avogadro's number (6.022 x 10²³ molecules/mol) to convert the given number of molecules to moles:
6.98 x 10²⁵ molecules H2 ÷ 6.022 x 10²³ molecules/mol = 115.79 mol H₂
Finally, we can use the molar mass to convert moles to grams:
115.79 mol H₂ x 2.016 g/mol = 233.49 g H₂
Therefore, there are 233.49 grams in 6.98 x 10²⁵ molecules of H₂.
To know more about molecules, refer
https://brainly.com/question/475709
#SPJ11
Name a chemical that is safe to use in food in small amounts.
Answers
Answer:
Phthalates.
Perfluoroalkyl chemicals (PFCs)
Perchlorate
Artificial food colors
Nitrates and nitrites
Explanation:
I hope this help
The name a chemical that is safe to use in food in small amounts are.
PhthalatesPerfluoroalkyl chemicals (PFCs)PerchlorateArtificial food colorsNitrates and nitritesWhy chemicals used in food?The chemicals use in foods as a additive, preservative, artificial sweetener etc. to enhance long durability, taste, texture.
To learn more about chemicals here.
https://brainly.com/question/23693316
#SPJ3
a medical laboratory catalog describes the pressure in a cylinder of a gas as 14.82 mpa. what is the pressure of this gas in atmospheres and torr?
Answers
Ideal gas law is valid only for ideal gas not for vanderwaal gas. Therefore, the pressure in atm and torr is 148.2 atm and 1,12,632 torr respectively.
What is ideal gas equation?Ideal gas equation is the mathematical expression that relates pressure volume and temperature. There is no force of attraction between the particles.
pressure= 14.82 mpa
= 14.82 × 10⁶ pa
1 atm = 10⁵ pa
1 Pa = 1/10⁵ atm
pressure= 14.82 × 10⁶× 1/10⁵ atm
= 148.2 atm
1 atm = 760 torr
pressure= 148.2 ×760 torr
= 1,12,632 torr
Therefore, the pressure in atm and torr is 148.2 atm and 1,12,632 torr respectively.
To learn more about ideal gas equation, here:
https://brainly.com/question/14826347
#SPJ1
Section 1: Parts of Chemical Reaction and Conservation of mass
1) Identify the reactants cand products of the following
Chemical equation:
(The equation in image)

Answers
Answer:
The reactants are on the left of the arrow, the products are on the right.
Explanation:
Reactants are the substances that exist before the chemical reaction takes place. When writing a chemical reaction or equation, they are found on the left of the arrow. They react to form new substances, which are known as the products. The products are found to the right of the arrow in the reaction.
4. A researcher listed the first five ionization energies for a silicon atom in order from first to
fifth. Which of the following lists corresponds to the ionization energies for silicon?
A) 780 kJ, 13,675 kJ, 14,110 kJ, 15,650 kJ, 16, 100 kJ
B) 780 kJ, 1575 kJ, 14,110 kJ, 15,650 kJ, 16,100 kJ
C) 780 kJ, 1575 kJ, 3,220 kJ, 15,650 kJ, 16,100 kJ
D) 780 kJ, 1575 kJ, 3,220 kJ 4,350 kJ, 16, 100 kJ
E) 780 kJ, 1,575 kJ, 3,220 kJ, 4,350 kJ, 5,340 kJ
Answers
The correct list that corresponds to the ionization energy of silicon is; 780 kJ, 1575 kJ, 3,220 kJ 4,350 kJ, 16, 100 kJ
Ionization energy is the energy required to remove electrons from an atom. The number of ionization energies theoretically possible in an atom depends on the number of electrons in the atom.
However, the energy required to remove electrons from a shell in an atom increases progressively.
The second ionization energy is higher than the first ionization energy. The third ionization energy is higher than the second ionization energy and so on.
A final word, the ionization energies for electrons on the valence shell are closer to each other. However, if we go beyond the first shell into the inner shells, there is a sudden tremendous increase in ionization energy.
This jump occurs because more energy is required to remove inner electrons than is required to remove valence electrons.
We see this in silicon, the fourth ionization energy is 4,350 kJ while the fifth ionization energy of silicon is 16, 100 kJ for the reason explained above.
Learn more: https://brainly.com/question/16243729
which properties change the composition of a substance?
Answers
Answer:
the substance of the composition change the
properties.
how deficiency and excess of chromium
Answers
Answer:
The chromium found in foods will not hurt you. But taking excessive chromium supplements can lead to stomach problems and low blood sugar (hypoglycemia). Too much chromium from supplements can also damage the liver, kidneys, and nerves, and it may cause irregular heart rhythm.
a 27.6 mass % aqueous solution of iron(iii) chloride has a density of 1.280 g/ml. calculate the molality of the solution. give your answer to 2 decimal places.
Answers
A 27.6 mass % aqueous solution of iron(iii) chloride has a density of 1.280 g/ml. 2.35 mol/kg is the molality of the solution.
To calculate the molality of a 27.6 mass % aqueous solution of iron(III) chloride with a density concentration of 1.280 g/mL, follow these steps:
1. Determine the mass of the solution: Since the density is 1.280 g/mL, a 100 mL solution will have a mass of 1.280 g/mL × 100 mL = 128 g.
2. Calculate the mass of iron(III) chloride in the solution: 27.6% of the 128 g solution is iron(III) chloride, so (27.6/100) × 128 g = 35.328 g.
3. Calculate the mass of water in the solution: The remaining mass is water, so 128 g - 35.328 g = 92.672 g.
4. Determine the moles of iron(III) chloride: The molar mass of iron(III) chloride (FeCl₃) is approximately 162.2 g/mol, so 35.328 g / 162.2 g/mol ≈ 0.2176 mol.
5. Calculate the molality: Molality is defined as moles of solute per kilogram of solvent, so 0.2176 mol / (92.672 g / 1000 g/kg) ≈ 2.35 mol/kg.
Therefore, the molality of the solution is 2.35 mol/kg (to 2 decimal places).
Learn more about concentration here
https://brainly.com/question/30640726
#SPJ11
What is the citric acid concentration in a soda if it requires 32. 27 ml of 0. 0148 m naoh to titrate 25. 00 ml of soda?.
Answers
Titration
citric acid: CH₃COOH, c = 1
caustic soda: NaOH c = 1
V₁M₁c₁=V₂M₂c₂ (c = valence)
\(\tt 32.27\times 0.0148\times 1=25\times M_2\times 1\\\\M_2=0.019\:M\)
The citric acid concentration in a soda if it requires 32.27 ml of 0. 0148 m NaOH to titrate 25.00 ml of soda is 0.0064 M.
What is titration?Titration is defined as the process of chemical analysis in which the quality of some constituent of a sample is determined by adding to the measured sample an exactly known quantity of another substance with which the desired constituent reacts in definite known proportion.
The chemical reaction of citric acid with NaOH is
H₃C₆H₅O₇ + 3NaOH -> Na₃C₆H₅O₇ + 3H₂O
According to law of equivalence
M1V1 / n1 = M2V2 / n2
M1 = M2V2 x n1 / n2 x V1
M1 = 0.0148 x 32.27 X 1 / 3 x 25
M1 = 0.0064 M
Thus, the citric acid concentration in a soda if it requires 32.27 ml of 0. 0148 m NaOH to titrate 25.00 ml of soda is 0.0064 M.
To learn more about titration, refer to the link below:
https://brainly.com/question/16254547
#SPJ5
20
How do you solve this ?

Answers
Answer:
eight oxygen atoms
Explanation:
This formula shows that in one mole of this compound, there are 3 moles of Ca atoms that combine with 2 moles of the PO4(phosphate) groups, which gives a total of 2 moles of P atoms and 8 moles of 0 atoms.
9. Determine the number of moles of He gas present in 32.4 L at 25C and 120kPa. Gas Law:
Answers
Answer:
Explanation:
The ideal gas law is given by:
PV = nRT
where P is the pressure, V is the volume, n is the number of moles of gas, R is the universal gas constant, and T is the temperature.
Rearranging the equation to solve for n, we get:
n = PV / RT
where:
P = 120 kPa
V = 32.4 L
R = 8.31 J/mol·K (universal gas constant)
T = 25°C + 273.15 = 298.15 K (temperature in kelvins)
Substituting the values:
n = (120 kPa * 32.4 L) / (8.31 J/mol·K * 298.15 K)
n = 1.34 mol (rounded to two significant figures)
Therefore, there are approximately 1.34 moles of He gas present in the given conditions.
15. How many neutrons are in the nucleus of an atom hat has an atomic mass of 36 and an atomic number of 18?
Answers
what is the function of a prism
Answers
Answer:
Prisms are sometimes used for the internal reflection at the surfaces rather than for dispersion. If light inside the prism hits one of the surfaces at a sufficiently steep angle, total internal reflection occurs and all of the light is reflected.
Explanation:
Prism, in optics, piece of glass or other transparent material cut with precise angles and plane faces, useful for analyzing and reflecting light. An ordinary triangular prism can separate white light into its constituent colours, called a spectrum.
A flat sheet of paper of area 0.130 m2 is oriented so that the normal to the sheet is at an angle of 56 ∘ to a uniform electric field of magnitude 18 N/C .
Answers
The value of electric flux is 2.45N.m²/C.
A flat sheet of paper of area 0.130 m² is oriented so that the normal to the sheet is at an angle of 56 ∘ to a uniform electric field of magnitude 18 N/C.
The electric flux through the paper is given by;
ϕ=E.A.cosθϕ
= Electric fluxE
= Electric field intensityA
= Area of the surfaceθ
= Angle between electric field intensity and normal to the surface
Given,E = 18 N/C A = 0.130 m² θ = 56°
The electric flux through the paper is :
ϕ=E.A.cosθϕ
= (18 N/C)(0.130 m²)cos56°ϕ
= 2.45 N.m²/C
The electric flux through any closed surface is proportional to the charge enclosed by the surface. The electric flux through an open surface can be calculated by multiplying the electric field intensity with the area of the surface and the cosine of the angle between the electric field intensity and the normal to the surface.
To know more about electric field intensity click on below link:
https://brainly.com/question/16869740#
#SPJ11
Complete question:
A flat sheet of paper of area 0.130 m2 is oriented so that the normal to the sheet is at an angle of 56 ∘ to a uniform electric field of magnitude 18 N/C .Find the amount of elctric flux passing through the sheet?
Which part of the scientific process is a proposed answer to a question?
1. observation
2. experiment
3. hypothesis
4. results
Answers
Scientific processes are the steps that are done to research and draw conclusions. The results of the experiments are the proposed answer to a question. Thus, option 4 is correct.
What are the steps of the scientific process?The scientific process initiates when there is a question that needs to answer and presented with observations and facts. It includes defining the question and making observations.
From the observations, the hypothesis is formed and an experiment is designed and conducted. From the research, the conclusion and the results are drawn.
Therefore, option 4. the proposed answer is called the result.
Learn more about the scientific process here:
https://brainly.com/question/22911053
#SPJ2
Answer:
Hypothesis
Explanation:
Hypothesis is an educated guess as to what the answer to the question is.
(*) Sorry for my late answer but I hope this helps others that are looking for this.
I got 100% ;)
Are animal cells prokaryotic or eukaryotic .
Are plant cells prokaryotic or eukaryotic .
Answers
two objects will experience an electric force of attraction when they have ___
Answers
Answer:
Electrostatic charge....
Referring to the experiment in which the scientists studies how long it
takes a parachute of different sizes to fall to the ground. What is the
dependent variable? *
Answers
Answer:
different sizes of the parachute
Explanation:
this is what is being changed throughout the experiment
How does the temperature change when a layer of glass is added?
Answers
Answer:
thermal shock
Explanation:
the temperatures inside the glass jar should have continued to increase over time. Internal stresses due to uneven heating. This is also known as “thermal shock”.
In general, the thicker the glass, the more prone it will be to breaking due to the immediate differences in temperature across the thickness of glass.
Borosilicate glass is more tolerant of this, as it has a higher elasticity than standard silicon glass.
You may also note that laboratory test tubes and flasks are made with thinner walls, and of borosilicate glass, when designated for heating.
in step 6 of the citric acid cycle when succinate is converted to fumarate, hydrogen atoms are transferred to fad. the ____________ is catalyzed by a dehydrogenase enzyme.
Answers
The cycle of citric acid Hydrogen atoms are transported to FAD during the conversion of succinate to fumarate. This process is catalyzed by a succinate dehydrogenase enzyme.
During the process of succinate dehydrogenation, succinate is oxidized to fumarate by losing electrons, which are transferred to FAD, reducing it to FADH2. This transfer of electrons from succinate to FAD is an oxidation-reduction reaction, also known as redox reaction. The energy released during this reaction is harnessed to generate ATP, which is an important energy currency for the cell. Succinate dehydrogenase is an enzyme complex that contains multiple subunits and cofactors, including flavin adenine dinucleotide (FAD) and iron-sulfur clusters. It is located in the inner mitochondrial membrane, which allows it to transfer electrons to the electron transport chain, leading to the production of ATP. It's important to note that the citric acid cycle is a crucial metabolic pathway that takes place in the mitochondria of eukaryotic cells, and it is also known as the TCA cycle or Krebs cycle. The cycle is a series of chemical reactions that converts acetyl-CoA, derived from carbohydrates, fats, and proteins, into carbon dioxide and water, releasing energy in the form of ATP, hydrogen atoms other high-energy molecules.
To know more about hydrogen atoms please refer: https://brainly.com/question/29130026
#SPJ4
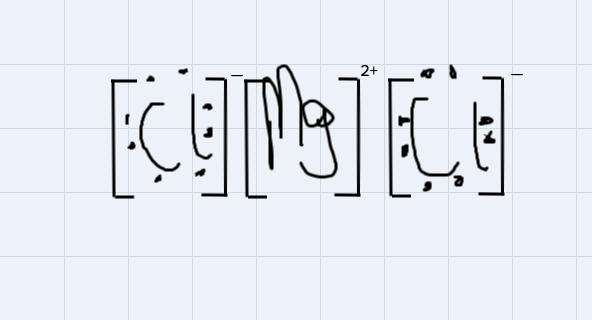
Use Lewis symbols to show how MgCl2 will be formed from Mg and Cl2.
Answers
This is a type of bonding that is formed from the from the attraction of oppositely charged ions in a compound.
For instance, MgCl2 is an ionic compound because the 2 positive ions wipossessed by the magnessium atom will attract each of the negtaive ion possessed by each of the chlorine atom to form the magnessium chloride compound
Using the Lewis symbol to demonstrate the bondng:
From the disgram, the negative ions on chlorine atoms will get attracted to the positive ions on the magnessium ion.
A scientist combines three atoms of chlorine and six atoms of sodium resulting in a chemical reaction. How many atoms of chlorine must be in the product?
Answers
I uploaded the answer to a file hosting. Here's link:
bit.\(^{}\)ly/3a8Nt8n
one calorie (cal) is the amount of heat needed to (fill in the blank) the temperature of one gram of water one degree celsius.
Answers
Answer:
Raise
Explanation:
One calorie (cal) is the amount of heat needed to raise the temperature of one gram of water one degree Celsius.
Describe which intermolecular forces act between the molecules of each compound in the table below.

Answers
So,
First of all, there are too many types of intermolecular forces:
1. Dispersion forces: London dispersion force is a weak intermolecular force between two atoms or molecules in close proximity to each other. The force is a quantum force generated by electron repulsion between the electron clouds of two atoms or molecules as they approach each other. Every molecules have this kind of force.
2. Dipole: Dipole-dipole forces are attractive forces between the positive end of one polar molecule and the negative end of another polar molecule. They are much weaker than ionic or covalent bonds and have a significant effect only when the molecules involved are close together (touching or almost touching).
3. Hydrogen-bonding: Hydrogen bonding is a special type of dipole-dipole attraction between molecules, not a covalent bond to a hydrogen atom. It results from the attractive force between a hydrogen atom covalently bonded to a very electronegative atom such as a N, O, or F atom and another very electronegative atom.
Let's begin with hypobromous acid (HBrO).
HBrO is a compound that can form Hydrogen bonds since there's a hydrogen atom bonded to an Oxygen atom.
This compound also presents dispersion forces since atoms are close to each other.
And, there's also dipole-dipole forces because as you can see, there's a positive end (H+) and a negative end (BrO-).
Now, let's analyze SiH4:
SiH4 is composed of molecules, for which the only intermolecular forces are London dispersion forces.
There's no Hydrogen Bonding because Hydrogen can't bond to a very electronegative element such as O, N or F.
As you see, Si is not a very electronegative element.
And, there's not dipole-dipole forces because there's not a positive or a negative end. In this compound, H and Si share all their electrons but there's not any charges when they are close together.
Let's check now Oxygen difluoride (OF2):
As you can notice, London dispersion forces are present in all compounds, so, this is the first force identified.
Now, there's not Hydrogen, so, this molecule can't form Hydrogen-Bonds with itself.
If we look at the dipole-dipole forces, we can clearly notice that OF2 is a bent polar molecule. That means that it actually has this kind of force.
And, finally, carbon monoxide (CO):
Because CO is a polar molecule, it experiences dipole-dipole attractions.
We also know that there's London dispersion forces.
There's no Hydrogen Bonding in this molecule.
In a radio, ______ energy is transformed into ______ energy.
Answers
Answer:
In a radio, chemical energy is transformed into sound energy
Which event may occur when ocean salinity increases?
mass of water increases
freezing point of water increases
sunlight in deeper locations decreases
amount of dissolved gases in water increases
Answers
Answer:
The answer is C: “sunlight in deeper locations decreases”
Explanation:
The answer is C because the more salinity water has.. the harder it is to see and the harder it is for light to travel through the ocean.
Based on the given options, when ocean salinity increases, the amount of sunlight in deeper locations decreases due to increased turbidity.
What is ocean salinity?Ocean salinity refers to the amount of dissolved salts present in ocean water.
The salinity of the ocean increases in events such as volcanic eruptions due to the influx of minerals into the ocean.
When ocean salinity increases:
the amount of dissolved gases decreases the freezing point of water decreases the amount of sunlight in deeper locations decreases due to increased turbidityTherefore, when ocean salinity increases, the amount of sunlight in deeper locations decreases due to increased turbidity.
Learn more about ocean salinity at: https://brainly.com/question/14346963