Answers
The solution's volume is 100 divided by 0.971 to get 102.98 mL. The number of moles of the solute (acetone) per litre of the solution is its molarity. Divide the mass by the molar mass to get the number of moles of acetone: 1.41 g / 58.08 g/mol = 0.0243 mol.
What is the molarity of the an aqueous solution's concentration?Molarity (M), which is determined by dividing the solute's mass in moles by the volume of the solution in litres, is the most widely used unit to express solution concentration: litres of solution/moles of solute equals M. One litre of a solution with a 1.00 molar concentration (1.00 M) contains 1.00 moles of solute.
What is the concentration calculation formula?The proportion of the solute that is dissolved in a solution is indicated by the solution's concentration. This formula can be used to determine a solution's concentration: Concentration is calculated as Volume of Solute multiplied by 100 and Volume of Solution (ml).
To know more about mass visit:
https://brainly.com/question/15959704
#SPJ1
Related Questions
When rain is in the forecast,
_____clouds are
likely to
appear
Answers
Answer: grey clouds
Explanation:
Answer:
Nimbostratus clouds if you want to be scientific but grey clouds ae also an answer.
Explanation:
Sorry if this late.
Sucrose (C12H22011) is combusted in air according to the following reaction:
C12H22011(s) + O2(g) = CO2(g) + H2O(l )
How many moles of carbon dioxide would be produced by the complete combustion of 38.5 grams of sucrose in the presence of excess oxygen?
Answers
Okay, let's break this down step-by-step:
1) The molecular formula for sucrose is C12H22011. This means each mole of sucrose contains 12 moles of carbon and 22 moles of hydrogen.
2) You are combusting 38.5 grams of sucrose. To convert grams to moles, we divide by the molar mass:
Molar mass of C12H22011 = 342.3 g/mol
So 38.5 g / 342.3 g/mol = 0.113 moles of sucrose
3) According to the combustion reaction, each mole of sucrose produces 12 moles of CO2.
So 0.113 moles of sucrose will produce 0.113 * 12 = 1.356 moles of CO2.
4) Round to the nearest whole number:
1 mole of CO2
Therefore, the complete combustion of 38.5 grams of sucrose in excess oxygen would produce 1 mole of carbon dioxide.
Let me know if you have any other questions!
Reduction of methanal equation
Answers
Answer:
hi
Explanation:
than you for points and thank me if you like
List the 2 pKa's for H2SO4
Answers
Help !
Student A uses 3N of force to move a cart 5 meters in 10 seconds. Student B uses 6N of force to move the same cart the same distance in 5 seconds. Which student did more work? Which student used more power? Use evidence and explain your answersss
Work = force x distance
Power = work/time
Answers
Work is said to be done if the force applied to a body causes the body to move through a distance.
Student B used more work and power
Workdone = Force * Distance
For student A:
Force = 3N
distance = 5meters
Time taken = 10 secs
Workdone by Student A = 3 * 5
Workdone by student A = 15Nm
Power used up = workdone/time
Power used up = 15/10
Power used up = 1.5 Watts
For student B:
Force = 6N
distance = 5meters
Time taken = 5 secs
Workdone by Student A = 6 * 5
Workdone by student A = 30Nm
Power used up = workdone/time
Power used up = 30/5
Power used up = 6 Watts
This shows that student B used more work and power
Learn more here: https://brainly.com/question/6701368
Scenario: You place a spoonful of frozen ice cream on your tongue a) Type of energy transfer b) Where will each form of transfer occur c) What will happen and why?
Answers
The type of energy transfer between the frozen ice cream and the tongue is convection. The frozen ice cream will gradually melt because of the temperature difference.
What is convection?Convection is the method of heat transfer that involves the transmission of heat in a fluid (liquid or gas) by the circulation of currents.
According to this question, a spoonful of frozen ice cream is placed on the tongue. The heat transfer will occur between the tongue with warmer temperature and the ice cream with colder temperature.
The heat energy will flow from the tongue to the ice cream, hence, making it defreeze or melt with time. This type of heat transfer is convection because it involves a fluid.
Learn more about convection at: https://brainly.com/question/4138428
#SPJ1
What is the z value for calcium atom with 35neutrons?
Answers
The atomic number of calcium i.e. Z value is 20 with 35 neutrons and 20 electrons.
What is atomic number?Atomic number is defined as a chemical element's nuclear charge number (symbol Z) is the charge number of an atomic nucleus.
It can also be defined as the number of protons or positive charges in the nucleus of an atom of a specific element, and thus the number of electrons generally surrounding the nucleus.
The number of protons is equal to number of number of electrons.
Thus. the atomic number of calcium i.e. Z value is 20 with 35 neutrons and 20 electrons.
To learn more about atomic number, refer to the link below:
https://brainly.com/question/8834373
#SPJ1
item 6 excessive intake of some vitamins can cause toxicity-related side effects. match the vitamin with the potential side effect when that vitamin is taken in excessive quantities.
Answers
Vomiting, headaches, wooziness, blurred vision, and even liver injury are side effects of vitamin A. Vitamin D - Tiredness, headache, sickness, and vomiting. Vitamin C: Heartburn, diarrhea, vomiting, and nausea.
What negative consequences can too much vitamin A cause?Yes, excessive amounts of some types of vitamin A can be dangerous. A strong headache, blurred vision, nausea, dizziness, muscular aches, and issues with coordination can result from taking too much preformed vitamin A, which is typically found in supplements or some medications.
Which vitamins can be harmful if consumed in large quantities?Poisonous Substance. Any component in a multivitamin supplement can be toxic in large doses, but iron or calcium pose the biggest threat. Large or toxic doses of calcium, vitamin D, and vitamin A carry additional dangers.
To know more about toxicity visit:-
https://brainly.com/question/21891087
#SPJ1
Differences between voltage, current and resistance?
Answers
Answer:
Voltage is the measure of electric potential energy per unit charge, current is the flow of electric charge through a circuit, and resistance is the property of a material that opposes the flow of electric current.
Ohm's Law relates these three concepts by stating that current is directly proportional to voltage and inversely proportional to resistance.
Hope this helps!
What kind of reaction is symbolized by AB + CD → AD + CB?
O A. Decomposition
OB. Synthesis
O C. Double replacement
OD. Single replacement
Answers
C. Double replacement
cations and anions switch places
Koja od navedenih atomskih orbitala nije moguća? a) 2d b) 3s c) 4p d) 5f
Answers
Answer:
2d
Explanation:
2d nije moguće. moguće je samo 3d do 7d. provjeri periodni sustav!
ako ne razumiješ, obrati mi pažnju
Question: What volume of 4.50 M HCI can be
made by mixing 5.65 M HCI with 250.0 mL of
3.55 M HCI?
Answers
Approximately 0.157 liters or 157 milliliters of the 4.50 M HCl solution can be made by mixing the given solutions.
To determine the volume of 4.50 M HCl that can be made by mixing the given solutions, we can use the concept of the concentration-volume relationship:
C1V1 = C2V2
Where:
C1 = concentration of the first solution
V1 = volume of the first solution
C2 = concentration of the second solution
V2 = volume of the second solution
Let's assign the variables as follows:
C1 = 5.65 M
V1 = unknown volume (we'll solve for this)
C2 = 3.55 M
V2 = 250.0 mL = 0.250 L (since the volume is given in milliliters)
Now we can plug in the values into the equation and solve for V1:
(5.65 M)(V1) = (3.55 M)(0.250 L)
Dividing both sides of the equation by 5.65 M:
V1 = (3.55 M)(0.250 L) / 5.65 M
V1 ≈ 0.157 L
For more question on solutions click on
https://brainly.com/question/25326161
#SPJ11
A type of weathering in which the surface of a rock is scratched and
physically worn away
Agent
Barrier island
Decay
Burrow
Abrasion
Answers
Answer:
Abrasion is the answer.
The weather balloon is inflated to a volume of 605dm3 at STP. The balloon is heated to 35 degree Celsius. What would be its volume at 75 cmHg?
Answers
The volume of the weather balloon at 75 cmHg is (n₁ * R * T₂) / P₂ = (n₁ * 0.0821 L·atm/(mol·K) * 308.15 K) / 0.9868 atm.
The volume of the weather balloon at 75 cmHg when it is heated to 35 degrees Celsius, we can use the ideal gas law, which states that the product of pressure (P) and volume (V) of a gas is proportional to the product of the number of moles (n) and the temperature (T) in Kelvin.
PV = nRT
Where R is the ideal gas constant.
First, let's convert the initial volume from dm³ to liters:
Initial volume = 605 dm³ = 605 L
At STP (Standard Temperature and Pressure), the temperature is 273.15 K and the pressure is 1 atmosphere (atm).
Using the initial conditions:
P₁ = 1 atm
V₁ = 605 L
T₁ = 273.15 K
We can calculate the number of moles (n₁) of gas using the ideal gas law:
n₁ = (P₁ * V₁) / (R * T₁)
Now, we need to find the final volume (V₂) at 75 cmHg and 35 degrees Celsius.
Converting 75 cmHg to atmospheres:
P₂ = 75 cmHg * (1 atm / 76 cmHg) ≈ 0.9868 atm
so,
P2 = 75 cmHg / 76 cmHg/atm = 0.9868 atm.
Now we can substitute the values into the equation and calculate V2:
V2 = (1 atm / 0.9868 atm) * (T2 / 308.15 K) * (605 liters).
Please provide the new temperature (T2) in Celsius, and I will calculate the volume for you.\((n₁ * R * T₂) / P₂ = (n₁ * 0.0821 L·atm/(mol·K) * 308.15 K) / 0.9868 atm\)
For more such questions on volume
https://brainly.com/question/27100414
#SPJ11
According to Bronsted-Lowry Theory, the conjugate base of HBr is.....Group of answer choicesH2Br+HBrBr-
Answers
answer and explanation
it is the conjugate of Br-
Plants use sunlight to produce some ATP during photosynthesis. How do plants produce ATP when the Sun is not out?
Plants are secondary consumers.
Plants also use cellular respiration.
Plants extract ATP from the stars.
Plants are weak in the dark.
Answers
Answer:
The answer is
Plant also use cellular respiration
Answer:
B - Plants also use cellular respiration.
Explanation:
Did the test.
What kind of substance is composed of two or more elements that are chemically combined?
Answers
Answer:
A molecule made of two or more elements chemically combined is a compound.
A molecule made of two atoms of the same element is just that an element.
A molecule made of two ( or more) elements is a compound.
Explanation:
Which part of this chemical process requires energy?
LiOH + HF
LiF + H20
O A. Forming bonds in LiOH and HF
O B. Breaking bonds in LiOH and HF
O C. Forming bonds in LiF and H20
O D. Breaking bonds in LiF and H20
Answers
Answer:
Breaking bonds in LION and HF
Explanation:
Breaking bonds in LiOH and HF requires energy in the given chemical process. Therefore, the correct option is option B.
What is bond dissociation energy?One indicator of a chemical bond A–B's strength is the bond-dissociation energy. It can be described as the typical enthalpy change that occurs when A and B, whose are typically radical species, are split apart by homolysis. The bond-dissociation energy is commonly described as the enthalpy change that occurs during homolysis at 0 K, while the enthalpy change at 298 K is additionally a frequently seen parameter.
The terms "bond-dissociation energy" and "bond-dissociation enthalpy," which are often used interchangeably, are comparable. The bond-dissociation energy (D0), according to some authors, actually refers to the shift in enthalpy at 0 K. Breaking bonds in LiOH and HF requires energy in the given chemical process.
Therefore, the correct option is option B.
To know more about bond dissociation energy, here:
https://brainly.com/question/13671588
#SPJ5
What is the mass in grams of 31.0 mL of gasoline?
Answers
Answer:
0.85g
Explanation:
This is a pretty straight forward equation, you would use PV=nRT to get this answer
Hope this helps!!!
31.0 mL of gasoline weighs 23.3 grams
Write the name or the formula of the following compounds:
a. (Co(ClO3)4(NO2))Cl (N in NO2 is underlined)
b. K(Co(SO4)(NH2CH2CH2NH2)2(CN)) (N in CN is underlined)
c. sodium carbonyldiisothriocyanatotrinitritochromate(III)
b. amminediaquatrihydroxidochromium(VI) nitrate
Answers
The molecular formula for a molecular compound's molecular formula lists the variety of atoms that make up the molecule. The name of the compound (Co(ClO₃)₄(NO₂))Cl is tetrachloridobis(nitrito-O)cobalt(III) chloride.
The name or the formula of the following compounds are:
The name of the compound (Co(ClO₃)₄(NO₂))Cl is tetrachloridobis(nitrito-O)cobalt(III) chloride.
The name of the compound K(Co(SO₄)(NH₂CH₂CH₂NH₂)₂(CN)) is potassium bis(ethylenediamine)dihydroxidocyanidocobaltate(III) sulfate.
The formula of the compound sodium carbonyldiisothriocyanatotrinitritochromate(III) is Na[Cr(NCS)₂(NO₂)₃(CO)].
The formula of the compound amminediaquatrihydroxidochromium(VI) nitrate is [Cr(OH)₄(NH₃)₂(H₂O)₂]NO₃.
Learn more about formula, here:
https://brainly.com/question/30062954
#SPJ1
Can someone teach me step by step how finding the oxidation number in this problem:
Fe in Fe(CIO2)3
Answers
Answer:
+3
Explanation:
u see sum of oxidation number in all situations have to be 0
ClO2 =-1
so Fe is +3
Below are pictures of a coastline and a river. Which of the following changes to the Earth's surface can be caused by wind and water
Answers
Answer: tsunami no cap
Explanation:
i need help with this, ive been trying to figure it out but i don’t understand. please number them 1-5 for the answers.

Answers
The solubility of the salts is affected by the temperature changes. 1. NaCl is least affected by temperature. 2. supersaturated. 3. 60 grams KBr. 4. Ethanol has both polar and non-polar groups. 5. Mixing and shaking.
A KBr solution with 90 gm solute in 100 grams of water at 50 degrees is classified as supersaturated. 60 grams of KBr are needed to make a saturated solution in 100 gm of water at 30 degrees.
Ethanol is a general solvent due to the presence of both the polar and the non-Polar groups. As a result, it is easier to dissolve both polar molecules and non-Polar molecules. The dissolving rate can be increased by mixing or shaking the solution. Also, the sugar dissolves faster in hot than cold tea.
To learn more about the solubility, refer to the link:
https://brainly.com/question/31493083
#SPJ1
Which has the greatest ionization energy?Group of answer choicesCHNOALL ARE SAME
Answers
Ionization energy is the minimum energy required to remove an electron from an isolated atom in the gaseous state.
The ionization energy of each one:
C - 1086
H - 1312
N - 1402
O - 1314
Answer: N has the greatest ionization energy.
Complete each row of the table below by filling in the missing pre
1 mol
1 M mol
1 m mol
1
mol
A
ha
10
10
= 10
= 10
0
0
-1
mol
mol
mol
mol
Н
X
5

Answers
Complete of row:
0.01 mol = 10⁻² mol1 M mol = 10³ mol1 m mol = 10⁻³ mol0,1 mol = 10⁻¹ molA mole is a unit of account for chemistry. The unit of account is used to facilitate the calculation of an object.
One mole of any substance will have the same number of particles, which is equal to 6.02 × 10²³ particles." For example, 1 mole of air contains 6.02 × 10²³ molecules of H₂O. 1 mole of oxygen gas contains 6.02 × 10²³ molecules of O.
The mole scale is needed as an indication of the amount of substance or compound, mole is the gram of substance divided by the relative molecular mass (Mr). The formula for calculating the moles of a compound is n = gram/Mr, in this case, n is the moles of the substance and gr is the mass of the substance.
Learn more about mole at https://brainly.com/question/29367909
#SPJ1
Question 3 (1 point)
Resistance is the slowing of the flow of electrons and where some of the electrical
energy is converted into heat.
Lesson 5.03
True
False
Answers
Answer:
False
Explanation:
The given statement stating that resistance is the slowing of the flow of electrons and where some of the electrical energy is converted into heat is true and it is a opposite of conduction.
What is conduction?
Conduction is defined as a process as a means of which heat is transferred from the hotter end of the body to it's cooler end.Heat flows spontaneously from a body which is hot to a body which is cold.
In the process of conduction,heat flow is within the body and through itself.In solids the conduction of heat is due to the vibrations and collisions of molecules while in liquids and gases it is due to the random motion of the molecules .
When conduction takes place, heat is usually transferred from one molecule to another as they are in direct contact with each other.There are 2 types of conduction:1) steady state conduction 2) transient conduction.According to the type of energy conduction is of three types:
1) heat conduction
2) electrical conduction
3)sound conduction
Learn more about conduction,here:
https://brainly.com/question/12136944
#SPJ5
For the diprotic weak acid H2A, a1=3.2×10−6 and a2=6.1×10−9 .
What is the pH of a 0.0750 M solution of H2A ?
What are the equilibrium concentrations of H2A and A2− in this solution?
Answers
In the first dissociation of H2A:
molarity H2A(aq)↔ (HA)^-(aq) + H^+(aq)
initial 0.05 m 0 m 0 m
change -x +x +x
equilibrium 0.05-x x x
we can neglect X in [H2A] as it so small compared to the 0.05
so by substitution in Ka equation:
Ka1 = [HA][H] / [H2A]
2.2x10^-6 = X^2/0.05
X = √(2.2x10^-6)*(0.05)= 1.1x10^-7
X= 3.32x10^-4 m
∴ [H2A] = 0.05 - 3.32x10^-4 = 0.0497 m
[HA] = 3.32x10^-4 m
[H] = 3.32x10^-4 m
the second dissociation of H2A: when ka2 = 8.2x10^-9
HA-(aq) ↔ A^2- (aq) + H+(aq)
at equilibrium 3.32x10^-4 y 3.32x10^-4
Ka2 = [H+][A^2-] / [HA]
8.2x10^-9 = Y(3.32x10^-4)/(3.32x10^-4)
∴y = 8.2x10^-9 m
∴[A] = 8.2x10^-9 m
PH= -㏒[H+]
= -㏒(3.32x10^-4)= 3.479
[A]=8.2x10^-9 m
[H2A] = 0.0497 ≈ 0.05 m
write the structural formula for 2-bromo-3-chloro-4,4-dimethylpentanal
Answers
Answer:
Br-CH2-CH(CH3)2-C(Cl)H-CH(CH3)2-CHO
Explanation:
The molecule has a total of 14 carbon atoms, 13 hydrogen atoms, and 1 bromine atom. The carbon atoms are arranged in a chain with a methyl group attached to the second carbon atom, a chlorine atom attached to the third carbon atom, and two methyl groups attached to the fourth carbon atom. The fifth carbon atom has a carbonyl group attached to it.
The molecule is an aldehyde, which means that it has a carbonyl group (C=O) at the end of the chain. The carbonyl group is polar, and the oxygen atom has a partial negative charge. The hydrogen atom has a partial positive charge. This polarity makes the aldehyde group susceptible to nucleophilic attack.
The bromine and chlorine atoms are both electrophilic, which means that they have a partial positive charge. This makes them susceptible to nucleophilic attack.
The methyl groups are non-polar and do not have any significant reactivity.
The molecule is a chiral molecule, which means that it has a mirror image that is not superimposable on itself. This is because the carbon atom with the carbonyl group is attached to four different groups.
The molecule is a liquid at room temperature and has a strong odor. It is used in a variety of products, including perfumes, flavorings, and plastics.
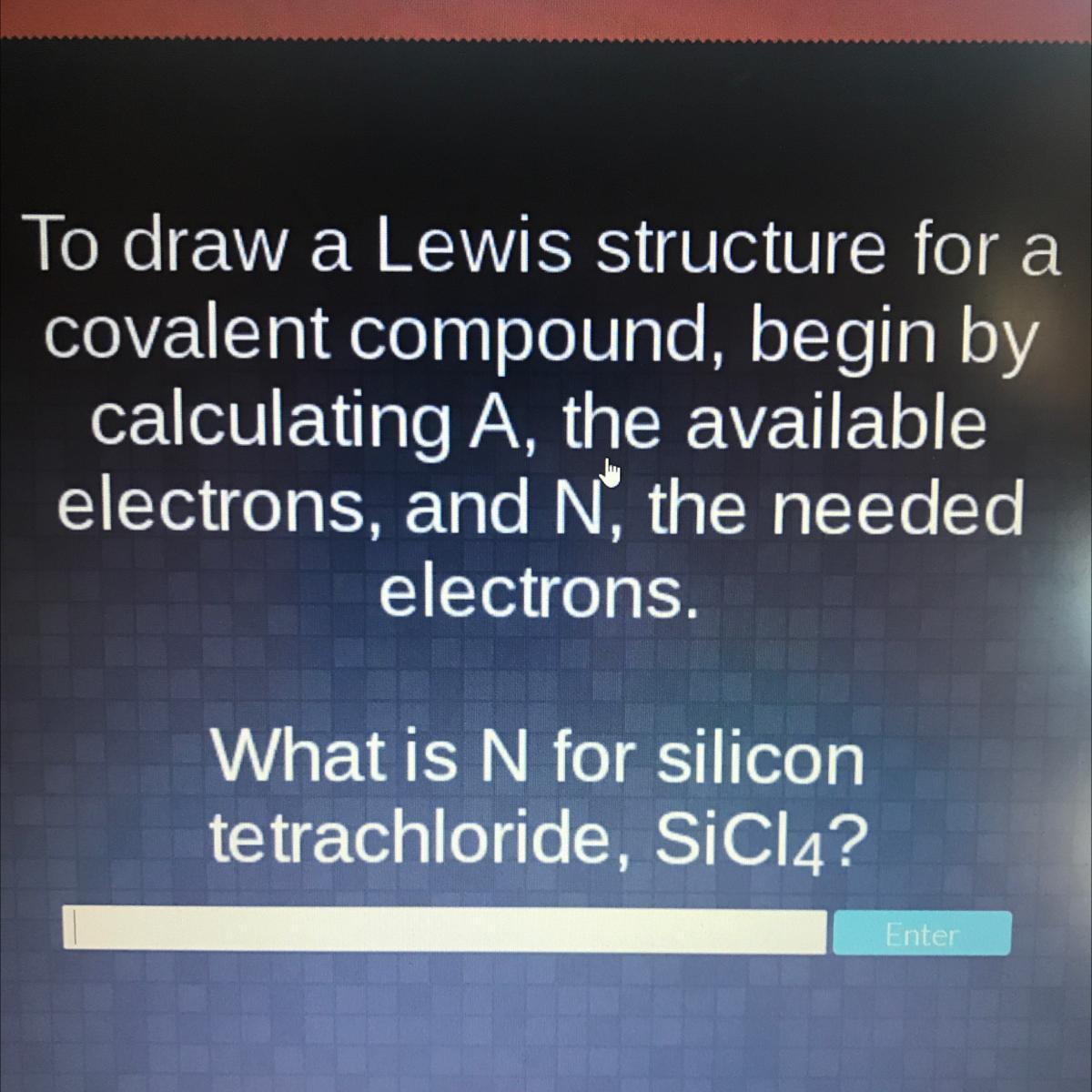
What is the answer to this?

Answers
What is N for silicon tetrachloride, SiCl4?