what is the change in the free energy, ∆g°, for the reaction? 4 kclo3(s) 3 h2s(aq) → 4 kcl(s) 3 h2so4(aq) substance ∆gf° (kj/mol) kclo3(s) -296.3 h2s(aq) -27.7 kcl(s) -408.5 h2so4aq() -744.6
Answers
∆G° = -2599.5 kJ/mol
The change in free energy (∆G°) for the reaction can be calculated using the formula:
∆G° = ∑(∆Gf° of products) - ∑(∆Gf° of reactants)
For the given reaction:
4 KClO3(s) + 3 H2S(aq) → 4 KCl(s) + 3 H2SO4(aq)
∆G° = [4(-408.5) + 3(-744.6)] - [4(-296.3) + 3(-27.7)]
∆G° = (-1634 - 2233.8) - (-1185.2 - 83.1)
∆G° = -3867.8 + 1268.3
∆G° = -2599.5 kJ/mol
The given chemical reaction is:
4 KClO3(s) + 3 H2S(aq) → 4 KCl(s) + 3 H2SO4(aq)
The standard free energy change (∆G°) of this reaction can be calculated using the formula:
∆G° = ∑(∆Gf° of products) - ∑(∆Gf° of reactants)
where ∆Gf° is the standard free energy change of formation of the compound at 298 K and 1 atm pressure.
First, we need to determine the standard free energy change of formation for each compound involved in the reaction. The values for these standard free energy changes of formation are usually available in tables or can be calculated using various methods.
The values of standard free energy change of formation at 298 K and 1 atm pressure for the compounds involved in the given reaction are:
KClO3(s): -408.5 kJ/mol
H2S(aq): -744.6 kJ/mol
KCl(s): -296.3 kJ/mol
H2SO4(aq): -813.3 kJ/mol (Note: The given reaction produces only 3 moles of H2SO4, so we need to multiply its ∆Gf° value by 3/2 to get the correct value for the reaction.)
Using these values, we can calculate the standard free energy change (∆G°) for the given reaction as follows:
∆G° = [4(-408.5) + 3(-744.6)] - [4(-296.3) + 3(-813.3 × 3/2)]
∆G° = (-1634 - 2233.8) - (-1185.2 - 83.1)
∆G° = -3867.8 + 1268.3
∆G° = -2599.5 kJ/mol
The negative value of ∆G° indicates that the given reaction is spontaneous under standard conditions (298 K and 1 atm pressure) and the products (KCl and H2SO4) are more stable than the reactants (KClO3 and H2S).
To learn more about energy, refer below:
https://brainly.com/question/1932868
#SPJ11
Related Questions
Please help thank you!

Answers
a compound has an empirical formula of C6H6NO. what is its molar formula, if its molar mass is 216.2 g/mol
Answers
Answer: C12H12N2O2
Explanation:
which is not a redox reaction?
a. Formation of ammonium sulphate from ammonia and sulphuric acid
b. formation of nitrogen monoxide from ammonia
c. formation of sulphuric acid from sulphur
d. formation of zinc from zinc sulphide
help me please
Answers
Answer:
D. formation of zinc from zinc sulphide
Explanation:
A redox reaction could be explained as an artificial reaction in which electrons are moved between two reactants partaking in it. This substitution of electrons can be recognized by examining the variations in the oxidation states of the reacting classes. The generation of hydrogen fluoride is an illustration of a redox reaction. We can crack the reaction down to investigate the oxidation and loss of reactants.
A rigid container of O2 has a pressure of 2551 mmHg at a temperature of 713 K. What is the pressure at 273 K?
Answers
Answer:977.34
Explanation:
divide 2551 and 273 to get 3.778. then multiply 3.78 by 273 to gry 977.34.
A small container of perfume is opened in a classroom. Soon every student in the room smells the perfume. Explain this in terms of molecules.
Answers
Answer: possibly diffusion
Explanation:
all particles are in motion unless at a certain degree so they'd spread throughout the room diluting as they continue to spread out.
Which best represents a physical change?
A. formation of a new substance
B. formation of a precipitate
C. condensation
D. bubbling
Answers
Answer:
The answer I would go with is A formation of a new substance.
Explanation:
This is because physical change includes changes of state. Some could be changing from a liquid to gas or solid to gas. B is not even an answer that should be considered because it doesn't fit into the category. C and D represents some of the examples of the process of physical change. Like boiling, bubbling, melting, or freezing. So the best would be A.
someone help please i cant figure this out
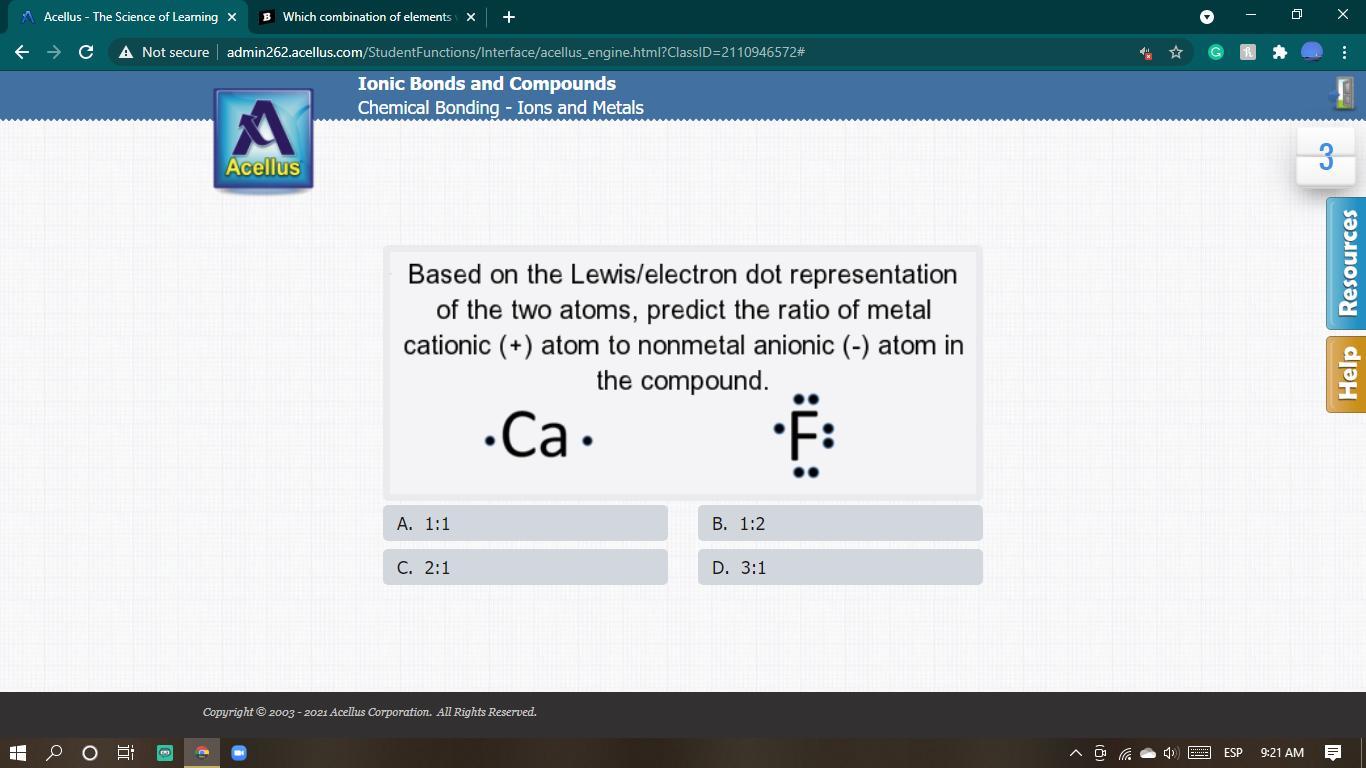
Answers
Answer: 2 : 1
Explanation:
Cation :
Ca - calcium = atomic number = 20
Electron dot configuration : 2, 8, 8, 2
Ca losses 2 electrons in its outermost shell and thus has a charge of 2+ in other to attain a stable octet state.
Anion:
F - has 7 valence electrons and thus needs 1 electron to achieve a stable octet state, hence it accepts one electron has has a charge of (-1)
Therefore,
Ratio of cation (+) to negative (-) = 2 :1
HELP ME PLEASE!! ASAP!!
Which of these pairs of elements is most likely to be part of a polyatomic ion?
Ba and O (Barium and Oxygen)
C and N (Carbon and Nitrogen)
K and Br (Potassium and Bromine)
Rb and Cl (Rubidium and Chlorine)
Answers
Answer:
Option B) Ba and O (Barium and Oxygen)
Explanation:
Barium and oxygen elements are the pairs which are most likely to be the part of polyatomic ion. As barium has +2 charge and Oxygen has -2 charge which will be settle once they form an polyatomic ion.
A person aims for a target with a wooden bow and arrow. The image shows the path of the arrow to the target. How can the person increase the elastic potential energy in this situation? A) aim the bow higher, increasing the height of the arrows flight. B) sharpen the arrows tip, causing it to strike the target with more force. C) change the bow’s material, increasing the stiffness of the bow. D) pull the arrow farther back, increasing the stretch of the bows string.
Answers
Answer:
d
Explanation:
How many grams in 1.000 moles of oxygen gas?
Answers
15 points
If calcium carbonate is heated strongly, carbon dioxide gas is driven off, leaving a
residue of calcium oxide. ((Write the unbalanced chemical reaction for this process))
Answers
If calcium carbonate is heated strongly, carbon dioxide gas is driven off , leaving a residue of calcium oxide then the unbalanced chemical reaction for this process is given as ,
\(CaCO_{3}\) → \(CO_{2}\) + CaO
A chemical equation that is out of balance has an uneven number of atoms from various elements in the reactants and products side . Chemical equations must be balanced since they are not accurately represented when they are unbalanced .
In balanced chemical equation the number of atoms in reactant side is equal to the number of atoms in product side .
to learn more about calcium carbonate please click here ,
https://brainly.com/question/13565765
#SPJ1
write the electron configurations for the elements that are identified only by these numbers
a. 15 b. 12 c. 9 d. 18
Answers
atomic number 15
1S^2,2S^2,2P^6,3S^2,3P^3
atomic number 12
1S^2,2S^2,2P^6,3S^2
atomic number 9
1S^2,2S^2,2P^5
atomic number 18
1S^2,2S^2,2P^6,3S^2,3P^6
Cordell bought new tires for his bicycle. As he rode his bike on the hot street, the temperature of the air in the tires increased. If the volume of the air stayed the same, what happened to the pressure inside the tires?
A. It decreased. B. It increased. C. It stayed the same. D. It was inversely proportional to the temperature
Answers
Answer: The answer is B. The pressure inside the tires increased.
Explanation:
The relationship between the pressure, volume, and temperature of a gas is described by the ideal gas law, which is usually written as:
\($$PV = nRT$$\)
where:
- \(\(P\)\) is the pressure,
- \(\(V\)\) is the volume,
- \(\(n\)\) is the number of moles of gas,
- \(\(R\)\) is the ideal gas constant, and
- \(\(T\)\) is the temperature (in Kelvin).
In this case, the volume \(\(V\)\) and the number of moles \(\(n\)\) of air in the tires stay the same. The temperature \(\(T\)\) is increasing. Therefore, for the equation to remain balanced, the pressure \(\(P\)\) must also increase.
So, the answer is B. The pressure inside the tires increased.
When the solution of substance X is added to a solution of potassium iodide, then a yellow solid separates out from the solution.a) What do you think substance X is likely to be?b) Name the substance which the yellow solid consists of.c) Which characteristics of chemical reactions is illustrated by this example?d) Write a balanced chemical equation for the reaction which takes place. Mention the physical states of all the reactions and products involved in the chemical equation.
Answers
a) The substance X is likely to be lead nitrate (Pb(NO₃)₂)
b) substance which the yellow solid consists of lead iodide (PbI₂).
c) The characteristic of chemical reactions that is illustrated by this example is the formation of a precipitate.
d) The balanced chemical equation for the reaction that takes place is:
Pb(NO₃)₂ (aq) + 2KI (aq) → PbI₂ (s) + 2KNO₃ (aq)
The material X is probably lead nitrate(Pb(NO₃)₂) since it interacts with potassium iodide (KI) to produce lead iodide (PbI₂), a yellow solid. Lead iodide is the yellow solid that separates from the solution (PbI₂).
The production of a precipitate is a property of chemical processes that is demonstrated by this example. A precipitate is a solid that develops during a chemical reaction from a solution. The reaction's balanced chemical equation is as follows:
Pb(NO₃)₂ (aq) + 2KI (aq) → PbI₂ (s) + 2KNO₃ (aq)
In this equation, the physical states of the reactants and products are indicated in parentheses. The aqueous solutions of lead nitrate and potassium iodide are indicated by (aq), and the solid lead iodide is indicated by (s). The aqueous solution of potassium nitrate is also indicated by (aq).
To learn more about chemical equation refer :
brainly.com/question/26227625
#SPJ11
A process in which substances chemically rearrange to form new substances
Molecule
Energy
Organism
Chemical reaction
Matter
Cellular respiration
Answers
Answer:
Chemical reaction
Explanation:
After new substances are formed they can't be reversed to their original state.
HELP MEEEEEE PLEASEEEEE WILL OFFER BRAINLIEST

Answers
Answer:
1.This answer would be option A.
2. I think that would be 1350
Explanation:
Guys please can you help me to do this please, if you see below there are two blue words, the first (north) can change to south and the second (south) can change to north and I need to know what the answer is

Answers
Answer: the earth is orbiting
Explanation: it changes and orbits
Atoms of which elements form bonds without satisfying the octet rule?
O potassium (K) and sodium (Na)
O neon (Ne) and argon (Ar)
• nitrogen (N) and fluorine (F)
• helium (He) and hydrogen (H)
Answers
Option (D) helium (He) and hydrogen (H) is the right answer.
The term "chemical compound" refers to a combination of two or more chemical elements, whether they are similar or dissimilar. For instance, the chemical compound H2O is made up of two oxygen atoms and one hydrogen atom.Ionic, covalent, and hydrogen bonds are the three basic types of chemical bonds that are used to generate these chemical compounds from their component atoms.Helium and hydrogen are exceptions to this octet rule because they do not require 8 electrons to finish their while forming the bond with another element, the valence shell.Most elements from the periodic table generally follow the octet rule when forming the bond with the other elements, which is to complete their outermost shell with eight electrons.Hence we can consider that Option(D)helium (He) and hydrogen (H) is the right answer.To learn more about octet rule visit:
https://brainly.com/question/865531
#SPJ9
Answer: Helium, Hydrogen
Explanation: I got it right
What is the calculated value of the cell potential at 298K for an electrochemical cell with the following reaction, when the Cu2+ concentration is 8.24×10-4 M and the Mn2+ concentration is 1.42 M ? Cu2+(aq) + Mn(s) Cu(s) + Mn2+(aq) Answer: ______V The cell reaction as written above is spontaneous for the concentrations given:
Answers
The calculated value of the cell potential at 298K for an electrochemical cell, \(Mn(s)/Mn^{2+}(1.42M)||Cu^{2+}(8.24 × 10^{-4}M)/Cu(s)\), is equals to the - 0.0868 V. It is true that the reaction is spontaneous for specify concentration.
We have an electrochemical cell with the following reaction, Cu²⁺ (aq) + Mn(s) --> Cu(s) + Mn²⁺ (aq)
Concentration of Cu²⁺ = 8.24 × 10⁻⁴ M
Concentration of Mn²⁺ = 1.42 M
Temperature, T = 298 K
There are 2 half-cell equations,
\(Cu^{2+}_{(aqu)}+2e^{-}\rightarrow Cu_{(s)}\)
The above one represents the reaction in the reduction half-cell(Cathode). The cell potential for this reaction is
\(E=E^{0}- \frac{0.0592}{n}log\frac{1}{[Cu^{2+}]}\)
where E⁰ is the standard electrode potential and is 0 for this cell (concentration cell with the same element as anode and cathode) and n is the number of electrons involved. Here, [Cu²⁺] = 8.24 × 10⁻⁴ M
\(E=0- \frac{0.0592}{2}log\frac{1}{[8.2 × 10^{-4}]}\)
= -0.09135V
Similarly for the oxidation half-reaction in the anode, \(Mn_{(s)}\rightarrow Mn^{2+}_{(aqu)}+2e^{-}\)
[Mn²⁺ ] = 1.42 M
\(E=0- \frac{0.0592}{2}log\frac{1}{[1.42]}\) = -0.00451 V
cell potential of the reaction can be calculated by the formula, \(E_{cell}=E_{cathode}-E_{anode}\)
= -0.09135 - (-0.00451)
= - 0.0868 V
Since Ecell < E⁰ the given reaction is spontaneous. Hence, reaction is spontaneous reaction.
For more information about cell potential, visit :
https://brainly.com/question/31965012
#SPJ4
Complete question:
What is the calculated value of the cell potential at 298K for an electrochemical cell with the following reaction, when the Cu2+ concentration is 8.24×10-4 M and the Mn2+ concentration is 1.42 M ? Cu2+(aq) + Mn(s) Cu(s) + Mn2+(aq) Answer: ______V The cell reaction as written above is spontaneous for the concentrations given: true/false.
Calculate mass, m in kg for one molecule of pentane. Report answer in scientific notation to 3 SF. Must show all proper units.
Answers
The mass of one molecule of pentane is approximately 7.22 x
\( {10}^{5} \)
kg when expressed on a per molecule basis.
To calculate the mass of one molecule of pentane, we need to know the molecular formula of pentane, which is C5H12.
The atomic mass of carbon is 12.011 g/mol, and there are 5 carbon atoms in pentane, so the total mass of carbon in one molecule of pentane is:
5 carbon atoms x 12.011 g/mol = 60.055 g/mol
The atomic mass of hydrogen is 1.008 g/mol, and there are 12 hydrogen atoms in pentane, so the total mass of hydrogen in one molecule of pentane is:
12 hydrogen atoms x 1.008 g/mol = 12.096 g/mol
Therefore, the total mass of one molecule of pentane is:
Total mass = Mass of carbon + Mass of hydrogen
Total mass = 60.055 g/mol + 12.096 g/mol
Total mass = 72.151 g/mol
We need to convert this to kilograms, which we can do by dividing by 1000:
72.151 g/mol ÷ 1000 g/kg = 0.072151 kg/mol
Finally, we can express this answer in scientific notation to three significant figures:
m =
\(7.22 x {10}^{5} kg/mol\)
learn more about mass here:
https://brainly.com/question/17067547
#SPJ1
Scientist repeats an experiment and gets a
different result. What should the scientist do next
Answers
Answer:
tty to fin out what happend by redoing it
Potassium and bromine combine to make KBr. What is the name of this compound?
potassium bromine
potassium bromide
bromine potassium
bromine potassiumide
Answers
why you do not observe e1 product in reaction of ethanol with bromotriphenylmethane.
Answers
E1 product is not observed in the reaction of ethanol with bromotriphenylmethane.
In the reaction of ethanol with bromotriphenylmethane, E1 product is not observed. Why?When an alcohol is reacted with HBr, it forms an alkyl halide. This is an example of nucleophilic substitution. The reaction follows an S_N1 or S_N2 mechanism, depending on the structure of the alcohol. E1 elimination is also possible, but it is rare. When ethanol reacts with HBr, it follows an S_N1 or S_N2 mechanism to form ethyl bromide. In this reaction, bromotriphenylmethane is reacted with ethanol to give triphenylmethanol and ethyl bromide as products.
The reaction takes place through an S_N1 mechanism. When the reaction occurs, the ethanol molecule replaces one of the bromine atoms on the bromotriphenylmethane molecule to create a new intermediate, which is more stable than the original compound. The intermediate has a cationic center with the positive charge on the central carbon atom (trityl carbon).
This trityl carbocation is highly stable because it is surrounded by three bulky phenyl groups that shield it from nucleophilic attack. The intermediate reacts with ethanol to form the desired products, triphenylmethanol and ethyl bromide. Therefore, E1 product is not observed in the reaction of ethanol with bromotriphenylmethane.
To learn more about ethanol visit;
https://brainly.com/question/29294678
#SPJ11
What allows us to convert from moles of one substance to moles of another substance?
A. Group of answer choices
B. Formula mass
C. Molecular mass
D. A balanced chemical equation
E. A conversion table
Answers
To convert from the moles of one substance to the moles of another substance the one that allows is the correct option is D. A balanced chemical equation.
The balanced equation is the the chemical equation in which the number of the moles of the atoms in the reactant side is equals to the number of the moles of the product side of each of the atom. From the balanced chemical equation we will find out the moles of the substance that involves in the chemical reaction.
Thus, from the balanced chemical equation we will convert the moles of the one substance to moles of the another substance.
To learn more about balanced equation here
https://brainly.com/question/8062886
#SPJ4
Since NAD+ and NADP+ are essentially equivalent in their tendency to attract electrons, discuss how the two concentration ratios might be maintained inside cells at greatly differing values.
Check all that apply.
1.Because NAD+-dependent enzymes usually act to dehydrogenate (oxidize) substrates, an [NAD+]/[NADH] ratio greater than unity tends to drive reactions in that direction.
2.[NADP+]/[NADPH] ratio less than unity provide concentrations that tend to drive these reactions in the direction of substrate oxidation.
3. Because NADH-dependent enzymes usually act to hydrogenate (oxidize) substrates, an [NAD+]/[NADH] ratio greater than unity tends to drive reactions in that direction.
4. Because NAD+-dependent enzymes usually act to hydrogenate (reduce) substrates, an [NAD+]/[NADH] ratio greater than unity tends to drive reactions in that direction.
5. [NADP+]/[NADPH] ratio less than unity provide concentrations that tend to drive these reactions in the direction of substrate reduction.
6. [NADP+]/[NADPH] ratio less than unity provide concentrations that tend to drive these reactions in the direction of enzyme oxidation.
Answers
NAD+ and NADP+ are important coenzymes in cellular metabolism, involved in redox reactions and energy transfer. While they are equivalent in their tendency to attract electrons, their concentrations inside cells are greatly different. One possible explanation for this is their distinct roles in different metabolic pathways.
For instance, NAD+ is mainly involved in catabolic processes, such as glycolysis and the citric acid cycle, while NADP+ participates in anabolic processes, such as fatty acid and nucleotide synthesis. As a result, the concentration ratio of [NAD+]/[NADH] tends to be higher than unity, which favors substrate oxidation, while the [NADP+]/[NADPH] ratio is less than unity, which favors substrate reduction.
Another possible explanation is the regulation of enzymes involved in their synthesis and degradation. For example, the rate of NAD+ biosynthesis can be controlled by the availability of its precursors, such as nicotinamide and tryptophan. In addition, the degradation of NADH and NADPH can be regulated by enzymes such as alcohol dehydrogenase and glucose-6-phosphate dehydrogenase, respectively. Overall, the maintenance of NAD+ and NADP+ concentrations in cells involves a complex interplay of metabolic pathways and enzyme regulation, which is essential for cellular function and homeostasis.
To know more about coenzymes visit:
https://brainly.com/question/29386956
#SPJ11
Forensic psychiatry focuses on the relationship between human behavior and criminal justice.
O True
O False
Answers
Hope this helps
What are the limitations of your model in explaining fusion?

Answers
The major factors influencing fusion consist of the required high temperature and high pressure which is not shown in the model and hence becomes one of its limitations.
Two major requirements for fusion are:
1) The energy from the high temperature allows the hydrogen atoms to overcome the electrical attraction between the protons. Temperatures of roughly 100 million Kelvin are necessary for fusion. Hydrogen is not a gas at these temperatures; it is a plasma. The high-energy state of matter known as plasma is one in which all atoms have had their electrons removed and are now free to move about. The sun's massive mass and the compression of that mass in the core caused by gravity allow it to reach these temperatures.
2) The hydrogen atoms are compressed together by high pressure. For Fusion, they need to be within 1x10-15 meters of one another. The sun compresses hydrogen atoms together in its core using gravity and its bulk. We must use strong magnetic fields, potent lasers, and ion beams to force hydrogen atoms together.
To learn more about fusion please visit-
https://brainly.com/question/12701636
#SPJ9
the equivalence point of any acid-base titration can be determined visually from a titration curve by finding the place where
Answers
Answer:
where the slope of the titration curve is the greatest
The titration curve can be used to identify the equivalency point of the titration.The volume of titrant is where the titration curve has the steepest slope.
How do you find the equivalence point on a titration curve?
The equivalency point for acid-base titrations can be identified quite quickly.A simple pH meter is used to measure the pH of the solution being titrated after different amounts of titrant have been introduced to create a titration curve.The curve can then be read to determine the equivalency point. The equivalency point is identified using thermometric titrimetry, which gauges the rate at which a chemical reaction alters temperature.The inflection point in this instance denotes the threshold at which an exothermic or endothermic process is equivalent. The pH of a solution during a titration is represented graphically by a titration curve.The equivalence point in a strong acid-strong base titration is reached when the moles of acid and base are equal, and the pH is 7. The [H+] and [OH] concentrations must be equal at some point to be considered the equivalency point.Just a little bit beyond that is the endpoint, where the indicator color totally changes and the pH shifts from acidic to basic, or vice versa. The precise halfway point between the reactions of the titrant and the acid in the buffer solution is known as the half equivalence point.Because the pKa of the acid and the pH of the solution are equal at the half equivalence point, finding this point is not too difficult. A weak-acid/strong-base titration will have an equivalent point at a somewhat basic pH.The reason for this is that while the acid is not nearly as strong and does not completely dissociate to neutralize each equivalent of the base, the base is stronger and dissociates to a greater extent.To learn more about acid base titration refer
https://brainly.com/question/23687757
#SPJ2
What is the difference between reactants and products?
Group of answer choices
A Reactants are substances that are combined to form products in a physical reaction. Products are the result of substances being combined in a chemical reaction.
B Reactants are substances that are combined to form products in a chemical reaction. Products are the result of substances being combined in a physicalreaction.
C none of the above
D Reactants are substances that are combined to form products in a chemical reaction. Products are the result of substances being combined in a chemical reaction.
Answers
The correct answer is D. Reactants are substances that are combined to form products in a chemical reaction. Products are the result of substances being combined in a chemical reaction.
Select all types of muscles.
Skeletal
ℍ
Cardiac
Stretch
Smooth
Answers
Answer:
Skeletal
Cardiac
Smooth
Explanation:
The body contains three types of muscle tissue: skeletal muscle, cardiac muscle, and smooth muscl