What is oxidized and what is reduced in this reaction? cu(s) 2agno3(aq) → cu(no3)2(aq) 2ag(s) cu is reduced and ag is oxidized. ag is reduced and cu is oxidized. no3 is oxidized and cu is reduced. ag is oxidized and no3 is reduced.
Answers
From the reaction equation, Cu is oxidized in the reaction while Ag is reduced.
What is a redox reaction?The term redox reaction has to do with a reaction in which a specie is oxidized and another is reduced.
Here the reaction equation is; Cu(s) + 2AgNO3(aq) → Cu(NO3)2(aq) 2Ag(s) Cu. We can see that Cu is oxidized in the reaction while Ag is reduced.
Learn more about reaction: https://brainly.com/question/17434463
#SPJ4
Answer:
this is your sign to take a small break and go drink some water
Explanation:
edge 2022

Related Questions
A student heats a sample of Copper (II) sulfate in a crucible and records the data shown in the table. What is the complete formula and name for the compound before heating?
Mass of empty crucible 128.10 g
Mass of crucible + sample before heating 152.00 g
Mass of crucible + sample after heating 147.60 g
Answers
Explanation:
Copper (II) sulfate is usually present as a hydrous state, which is of the form CuSO4 * nH2O, where n is a whole number.
Mass of sample (CuSO4 * nH2O)
= 152.00g - 128.10g = 23.90g.
Mass of water loss during heating
= 152.00g - 147.60g = 4.40g.
Molar mass of H2O = 18g/mol
Moles of H2O in sample
= 4.40g / (18g/mol) = 0.244mol.
Mass of anhydrous sample (CuSO4)
= 23.90g - 4.40g = 19.50g
Molar mass of CuSO4 = 159.61g/mol
Moles of CuSO4 in sample
= 19.50g / (159.61g/mol) = 0.122mol.
Since mole ratio of CuSO4 to H2O
= 0.122mol : 0.244mol = 1:2, n = 2.
Hence we have CuSO4 * 2H2O.
Answer:
Copper(II) Sulphate (CuSO4.7H2O) is generally present in a hydrated form.
Mass of the CuSO4.7H2O sample before heating
(152.00-128.10)g = 23.90 grams
Mass of the water loss due to heating
(152.00-147.60)g = 4.40 grams
A gas at a constant pressure occupies 2.24 L at 52 C. If the sample is cooled United it takes up 1.66 L what is the new temperature?
A. 0.01 K
B. 0.01 C
C. 32 C
D. 241 K
PLS SHOW WORK
Answers
Answer:
D is the answer!Explanation:
Using Charles's Law to solve this question: V1/T1 = V2/T2
V1 = 2.24L
T1 = 52C (covert to Kelvin by plus 273.15) = 325.15K
V2 = 1.66L
T2 = ?
V1/T1 = V2/T2 must change to V2×T1/V1 = T2
1.66 x 325.15/2.24 = V2
V2 = 240.96K ≈ 241K
in what molecules does the presence of nonbinding electron pairs produce an effect on molecular shape
Answers
The presence of nonbonding electron pairs, also known as lone pairs or nonbonding electron domains, can have an effect on the shape of molecules. These lone pairs influence the molecular geometry by exerting electron repulsion and affecting the arrangement of atoms and bonding pairs.
Molecules that commonly exhibit the influence of nonbonding electron pairs on molecular shape include:
Water (H2O): In water, the two lone pairs of electrons on the oxygen atom affect the molecular shape, leading to a bent or V-shaped geometry.
Ammonia (NH3): Ammonia has one lone pair of electrons on the nitrogen atom, which leads to a pyramidal shape.
Nitrogen trifluoride (NF3): NF3 has one lone pair of electrons on the central nitrogen atom, resulting in a trigonal pyramidal shape.
Carbon dioxide (CO2): Although carbon dioxide does not possess any lone pairs on the carbon atom, the presence of two double bonds results in a linear molecular shape.
Sulfur hexafluoride (SF6): The six lone pairs of electrons on the sulfur atom in SF6 cause electronic repulsion, resulting in an octahedral shape.
These are just a few examples, but there are many molecules where nonbonding electron pairs influence the overall molecular shape. The presence and arrangement of these lone pairs affect the bond angles and distortions from ideal geometries in molecules, ultimately determining their three-dimensional shapes.
learn more about electron pairs here
https://brainly.com/question/29427403
#SPJ11
Commercial agriculture can often lead to water-quality problems. In one to two sentences, explain how two of those problems occur.
anyone ?????
Answers
Commercial agriculture can lead to water-quality problems because water can be used for irrigation and washing chemical products.
What is commercial agriculture?The term commercial agriculture makes reference to the techniques to produce high yields in extensive crops.
Commercial agriculture may lead to contamination problems due to the use of herbicides and pesticides.
In conclusion, commercial agriculture can often lead to water-quality problems because water can be used for irrigation and washing chemical products.
Learn more about commercial agriculture here:
https://brainly.com/question/1096916
#SPJ1
8. When 50.0 g of MgCO3 react completely with H3PO4, as shown below, 15.8 g of CO₂ is produced.
Determine the theoretical and percent yield for this reaction?
2 H3PO4 + 3 MgCO3 → Mg3(PO4)2 + 3 CO2 + 3 H₂O
Answers
The theoretical yield for this reaction is 78.61 g of CO2 and the percent yield is 20.0%, when 50.0 g of MgCO3 react completely with H3PO4.
What is theoretical yield?The theoretical yield is the maximum amount of product that can be produced from a chemical reaction, calculated using the stoichiometry of the reaction equation. It is based on the assumption that the reaction proceeds perfectly, without any loss or waste of reactants, and without any interference from side reactions.
The theoretical yield is calculated by determining the number of moles of each reactant, using the balanced chemical equation to calculate the number of moles of product that can be produced, and then converting this to the corresponding mass of product. It is a useful reference for determining the efficiency of a reaction and for evaluating the quality of the results obtained in a laboratory experiment.
Calculation of Theoretical yield
To calculate the theoretical yield, determine the number of moles of MgCO3 and use the balanced chemical equation to calculate the number of moles of CO2 produced.
The number of moles of MgCO3 is given by:
n(MgCO3) = m(MgCO3) / M(MgCO3)
n(MgCO3) = 50.0 g / 84.31 g/mol
n(MgCO3) = 0.595 mol
Using the balanced chemical equation, calculate the number of moles of CO2 produced:
n(CO2) = 3 * n(MgCO3)
n(CO2) = 3 * 0.595 mol
n(CO2) = 1.785 mol
The mass of CO2 produced can then be calculated:
m(CO2) = n(CO2) * M(CO2)
m(CO2) = 1.785 mol * 44.01 g/mol
m(CO2) = 78.61 g
This is the theoretical yield for the reaction.
The percent yield is a measure of the efficiency of the reaction, and is calculated as the ratio of the actual yield to the theoretical yield, multiplied by 100:
% yield = (actual yield / theoretical yield) * 100
% yield = (15.8 g / 78.61 g) * 100
% yield = 20.0%
To know more about Theoretical Yield, visit:
https://brainly.com/question/25996347
#SPJ1
Which of these is a reason why nuclear power is important?
4.4.2 nuclear power
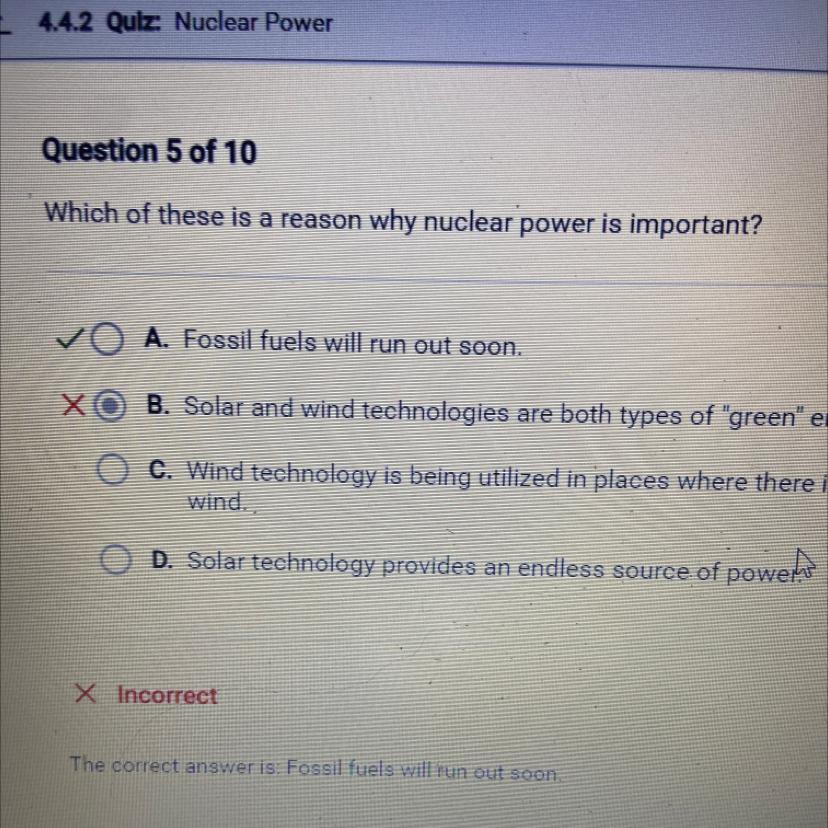
Answers
Answer:
Fossil fuels will run out soon\identify which reaction is likely to have the greatest positive enthalpy change. p(g) 5cl(g)→pcl5(g) p(g) 3cl(g)→pcl3(g) pcl3(g)→p(g) 3cl(g) pcl5(g)→p(g) 5cl(g)
Answers
The greatest positive enthalpy change has PCl₅(g) → P(g) + 5Cl(g)
An enthalpy change is difference between the enthalpy after the chemical reaction has completed.
An enthalpy change of phosphorus(V) chloride (PCl₅) molecule is energy needed for breaking up one molecule of phosphorus(V) chloride into one phosphorus and five chlorine atoms.
In a chemical reaction, chemical bonds are forming and breaking and this cause the transformation of one group of chemical substances to another.
Energy is needed to break chemical bonds in molecules (positive enthalpy change), while during formation of molecule, energy is released (negative enthalpy change)
The stronger are the bonds, more energy is needed and enthalpy change is more positive.
PCl₅ has more bonds to break than PCl₃.
More about enthalpy change: brainly.com/question/16387742
#SPJ4
what are metals in chemistry
Answers
usually referring to chemical elements that are solid
pononium ,and silver
Explanation:
hope it helped
the most stable (tightly bound) atomic nucleus in the universe is:
Answers
The most stable (tightly bound) atomic nucleus in the universe is the nucleus of iron-56 (56Fe). It has the highest binding energy per nucleon among all known elements.
Nuclear stability is determined by the balance between the strong nuclear force, which holds the nucleus together, and the electrostatic repulsion between protons, which tends to push the nucleus apart.
The binding energy per nucleon is a measure of the stability of a nucleus, indicating the amount of energy required to separate a nucleon from the nucleus.
Iron-56 has the highest binding energy per nucleon compared to other elements.
This means that, on average, the nucleons in iron-56 are more tightly bound than in any other nucleus. As a result, iron-56 is the most energetically favorable configuration, and any deviation from this configuration tends to release energy, either through fusion or fission reactions.
The stability of iron-56 is crucial for stellar nucleosynthesis, as it represents a key endpoint in the fusion processes occurring in the cores of massive stars.
It also plays a significant role in determining the relative abundance of elements in the universe.
Learn more about binding energy here:
https://brainly.com/question/32066931
#SPJ11
HELLPPPPP MEEEEEE
The following equation represents which of the following types of reaction?
4 H3PO4 ---> P4 + 5 O2 + 6 H2O
Select one:
a. Decomposition.
b. Double replacement.
c. Single replacement.
d. Synthesis.
Answers
The products and the reactants are the components of the reaction. The equation of phosphoric acid represents the decomposition reaction as it forms oxygen and water. Thus, option a is correct.
What is a decomposition reaction?A decomposition reaction is a chemical breakdown of the reactant into two or more products. The large and complex compound breaks into smaller and simpler molecules.
The following equation, 4 H₃PO₄ → P₄ + 5 O₂ + 6 H₂O is an example of decomposition as phosphoric acid breaks into phosphorus, water, and oxygen molecules.
Therefore, option a. the reaction of phosphoric acid is a decomposition reaction.
Learn more about decomposition reaction here:
https://brainly.com/question/24936069
#SPJ1
What is the function of the outer covering of a plant? a plant's outer covering is involved in reproduction. a plant's outer covering helps reduce water loss. a plant's outer covering is for preying on other plants. a plant does not have an outer covering.
Answers
Answer:
i dont know but good luck beacuse im doing the test and im stuck ngl
Explanation:
Answer:
B
Explanation:
What tuberculosis means "
Answers
Answer:
it is a infectiousr bacterial disease characterized by the growth of nodules(tubercles) in tissues especially the lungs
How many moles of water (H2O) are needed to react completely with 7. 3 moles of iron (Fe)? *
2 points
5. 5 mol water
2. 4 mol water
4. 0 mol water
9. 7 mol water
Answers
D. 9. 7 mol of water are needed to react completely with 7. 3 moles of iron (Fe)
To determine the number of moles of water needed to react completely with 7.3 moles of iron (Fe), we need to balance the chemical equation for the reaction between iron and water. The balanced equation is:
3 Fe + 4 \(H_{2}O\) -> \(Fe_{3}O_{4}\) + 4 \(H_{2}\)
According to the balanced equation, 4 moles of water are required to react with 3 moles of iron. This means that the stoichiometric ratio between water and iron is 4:3.
Given that we have 7.3 moles of iron, we can use this ratio to calculate the amount of water needed. We set up the following proportion:
4 moles \(H_{2}O\) / 3 moles Fe = x moles \(H_{2}O\) / 7.3 moles Fe
Cross-multiplying and solving for x, we find:
x = (4 moles \(H_{2}O\) / 3 moles Fe) * 7.3 moles Fe
= 9.73 moles \(H_{2}O\)
Therefore, approximately 9.7 moles of water are needed to react completely with 7.3 moles of iron. The closest option provided is 9.7 mol water. Therefore, Option D is correct.
The question was incomplete. find the full content below:
How many moles of water (\(H_{2}O\)) are needed to react completely with 7. 3 moles of iron (Fe)? * 2 points
A. 5. 5 mol water
B. 2. 4 mol water
C. 4. 0 mol water
D. 9. 7 mol water
Know more about mole here:
https://brainly.com/question/29367909
#SPJ8
What do endocytosis and exocytosis have in common?
13. What do endocytosis and exocytosis have in common?
A: Both processes require no energy to transport particles.
B: Both processes are much more efficient than active transport.
C: Both processes involve photosynthesis in order to generate energy.
D: Both processes involve the folding of the cell membrane.
Answers
Answer: the answer is D which is “Both processes involve the folding of the cell membrane”
The reaction was run with 23.5 g LiOH and an excess of potassium chloride. 18.85 g LiCl was produced. What is the percent yield for this run of the reaction?
Answers
If the reaction was run with 23.5 g LiOH and an excess of potassium chloride. 18.85 g LiCl was produced. 45.3% is the percent yield for this run of the reaction.
Thus, (Actual yield / Theoretical yield) x 100 is a formula for calculating the reaction's percent yield. With 18.85 g of LiCl produced and a theoretical yield of 41.58 g based on stoichiometry, the actual yield is around 45.3%. This shows that the conversion of LiOH to LiCl occurred with a modest degree of efficiency.
With a percent yield of around 45.3%, the reaction converted LiOH to LiCl with a mediocre level of efficiency. The reduced yield might be caused by elements like an incomplete reaction, adverse reactions, or loss during purification. LiOH is totally consumed when there is too much potassium chloride present, but maximal LiCl generation is not ensured.
Learn more about the potassium chloride here:
https://brainly.com/question/31104976
#SPJ1
PLEASE HELP ILL GIVE YOU 50 PTS AND BRAINLIEST
How many molecules (x 1021 or 1022--enter the significant digits) are present in 1.00 grams of Al(C2H3O2)2?
Answers
Answer:
602.2*10^21 and 60.22*10^22 respectively
Explanation:The molar mass for Al(C2H3O2) is 145.028G/ML. One mole of anything will contain 6.022* 10^23 molecules. If you are simply looking to express your answer using an exponent of 21 or 22 the answer would be 602.2*10^21 and 60.22*10^22 respectively. Hope this helps respectively. Hope this helps!
the empirical formula for the two compounds that have very diffrent properties is ch2). if the molar mass of compound a is 60.05 g/mol and compound b is 180.16 g/mol, what are the molecular formulas for these compounds?
Answers
The molecular formula of compound A is C₂H₅., the molecular formula for compound B is C₇H₁₄.
What is empirical formula?Empirical formula is the most straightforward formula for a compound and provides the ratio of various atoms found in each compound molecule.
The empirical formula for the two compounds is CH₂. To determine the molecular formula, we need to know the molar mass of the compounds.
If the molar mass of compound A is 60.05 g/mol, and the empirical formula is CH₂, we can calculate the molecular formula by dividing the molar mass by the molar mass of the empirical formula.
60.05 g/mol / (12.01 g/mol + 2 x 1.01 g/mol) = 2.5
Therefore, the molecular formula for compound A is C₂H₅
Similarly, if the molar mass of compound B is 180.16 g/mol, and the empirical formula is CH₂, we can calculate the molecular formula by dividing the molar mass by the molar mass of the empirical formula.
180.16 g/mol / (12.01 g/mol + 2 x 1.01 g/mol) = 7.5
Therefore, the molecular formula for compound B is C₇H₁₄.
To know more about Empirical formula ,visit :
https://brainly.com/question/14044066
#SPJ4
3. How many formula units of NaOH are in 0.87 moles?
Answers
Answer: 1 mole of H2O= about 1/3 of a cup (18 mL). It is helpful ... 6.02 x 1023 H2O molecules. = 6.02 x 1023 NaCl formula unit. 1 mole C. 1 mole H2O. 1 mole
Explanation:
What is the mass of 18.5 moles of iron?
Answers
Answer:
1 mole =56 g
18.5 moles =56×18.5
=1036 moles Hope this helps you Stay happy and safe Do mark as brainliest ✌️At which location are metamorphic rocks most likely to
form?
ОА
ов
с C
OD
the answer is B
Answers
Answer:
Yup B
Explanation:
WILL GIVE BRAINLIEST AND 20 POINTS How many grams are in 2.36 L of neon gas
Answers
Answer:
2.088464 grams.
Explanation:
The density of neon is 0.9002 g/L. Multiplying this value by the number of liters gives how many grams of neon it contains.
0.9002(2.32) = 2.088464
Liquid nitrogen, which has a boiling point of −195.79°C, is used as a coolant and as a preservative for biological tissues. Is the entropy of nitrogen higher or lower at −200°C than at −190°C? Explain your answer. Liquid nitrogen freezes to a white solid at −210.00°C, with an enthalpy of fusion of 0.71 kJ/mol. What is its entropy of fusion? Is freezing biological tissue in liquid nitrogen an example of a reversible process or an irreversible process?
Answers
Answer:
Explanation:
Entropy is measure of disorder so as we lower the temperature of gas , its entropy decreases .
Hence at - 200°C entropy of nitrogen will be less than that at - 190°C .
At freezing point ,
entropy of fusion = latent heat / freezing temperature
= .71 kJ / ( 273 - 210 )
= 710 / 63 J mol⁻¹ K⁻¹ .
= 11.27 J mol⁻¹ K⁻¹ .
entropy of fusion = 11.27 J mol⁻¹ K⁻¹ .
Please help me with this question, I already did part a and part b. Can someone please tell me how to do part c, d, e, f, g, and h. If you don't know the answer to all parts then just tell me the answer to the parts you know, and if you can also explain it will be very helpful

Answers
Answer:
Most Medicare Advantage plans also fold in prescription drug coverage. Not all of thesef, g, and h. If you don't know the answer to all parts then just tell me the answer to the parts you know, and if you
The best way to clean any computer component or device is to follow the manufacturer's instructions in your user manual.
Always power off the system first.
Always use anti-static wristband or other professional grounding devices.
Never work on carpeted surfaces.
Please help me with this question, I already did part a and part b. Can someone please tell me how to do part c, d, e,
Which sequence represents the relationship between temperature and volume as explained by the kinetic-molecular theory?
higher temperature, more kinetic energy, more space between particles, higher volume
higher temperature, less kinetic energy, less space between particles, higher volume
higher temperature, more kinetic energy, less space between particles, lower volume
higher temperature, less kinetic energy, more space between particles, lower volume
Answers
Answer:
higher temperature, more kinetic energy, more space between particles, higher volume
This is true because increase in temperature kinetic energy increase so molecules move apart and increase volume
Explanation:
If 46g of HC4 react with 32g of O2
CH4+2O2=CO3+2H2O
Answers
The given chemical equation can be balanced as: CH4 + 2O2 → CO2 + 2H2OTherefore, the balanced chemical equation for the reaction between 46 g of CH4 and 32 g of O2 is:CH4 + 2O2 → CO2 + 2H2OMoles of CH4 = (46 g)/(16.04 g/mol) = 2.87 molMoles of O2 = (32 g)/(32 g/mol) = 1 mol.
Moles of CH4 = (46 g)/(16.04 g/mol) = 2.87 molMoles of O2 = (32 g)/(32 g/mol) = 1 molFor the given reaction, one mole of CH4 reacts with 2 moles of O2. Therefore, 2.87 moles of CH4 would react with 2 × 2.87 = 5.74 moles of O2. As we can see from the given values, only 1 mole of O2 is present. Hence, O2 is the limiting reactant.The balanced chemical equation for the reaction between 46 g of CH4 and 32 g of O2 is:CH4 + 2O2 → CO2 + 2H2OOn
The basis of the balanced chemical equation, 2 moles of O2 is required for one mole of CH4. Here, the moles of O2 is lesser than 2 times the moles of CH4, which means O2 is the limiting reactant and CH4 is the excess reactant. Hence, 32 g of O2 will react completely with 1 mole of CH4, and 14.14 g of CO2 and 4 g of H2O will be produced as per the stoichiometry.
To know more about chemical equation visit:
https://brainly.com/question/28792948
#SPJ11
Earth’s ______________ (g) is nearly the same at any point on its surface.
The value of g near Earth’s surface is ______ meters per second squared (_______ m/s2).
If m is the mass of an object and g is gravity, then we can find the weight, W, of the object by using the
formula W = ______.
The weight of an object is approximately equal to _____ ______________ if the mass of the object
is 1 kilogram on Earth, where g = ________ m/s2 fill in the blank.
Answers
Answer:
Earth’s ____gravity_____ (g) is nearly the same at any point on its surface.
The value of g near Earth’s surface is _ten__ meters per second squared (__10 (preciesly it is 9.8)___ m/s2).
If m is the mass of an object and g is gravity, then we can find the weight, W, of the object by using the
formula W = __m×g__.
The weight of an object is approximately equal to _g__ ______________ if the mass of the object is 1 kilogram on Earth, where g = __10 (9.8)___ m/s2 fill in the blank
Explanation:
The physical state of CaBr2 at room temperature? Predict the two other properties of CaBr2 Deduce the charge of ion Ca in the compound CaBr2
Answers
Answer:
CaBr2 is a colorless solid and the charge on Calcium is +2
Explanation:
CaBr2 is a colorless solid at room temperature.
Other properties include -
a) It is a crystalline solid
b) Melting point of 765°C.
c) It is deliquescent
d) Soluble in water and absolute alcohol.
The charge of Br is -1. Here two atoms of Br attaches with one atom of calcium. Hence the charge of calcium is +2
A 150. gram sample of an unknown metal went from an initial temperature of 22.4°C to a
final temperature of 12.6°C. The sample underwent a change in thermal energy of -662 J. If the
sample is one of the metals listed in the table above, what is the identity of the metal?
Answers
Specific heat capacity if the unknown metal is -0.450 J/(g°C).
What is specific heat capacity?The measure of heat complexity needed to increase the temperature of a single unit of substance mass by one degree Celsius is known as specific heat capacity. This factor is crucial in determining how much energy is required for temperature changes in a given substance.
Equation:q = mcΔT
where q is the change in thermal energy, m is the mass of the metal, c is its specific heat capacity, and ΔT is the change in temperature.
In this case, we have:
m = 150 g
ΔT = 22.4°C - 12.6°C = 9.8°C
q = -662 J
Plugging in the values,
-662 J = (150 g) c (9.8°C)
Solving for c, we get:
c = -662 J / (150 g × 9.8°C) = -0.450 J/(g°C)
To know more about specific heat capacity, click here
https://brainly.com/question/29766819
#SPJ1
what type of land use can result in nutrient depletion
Answers
Which formula represents an ionic bond? 1 NaCl 2 N2O 3 HCl 4 H2O
Answers
Answer:
1 NaCl
Explanation:
NaCl is sodium chloride, and sodium chloride is an ionic compound because it's held together with ionic bonds.