Answers
Answer: Fluid molecules move quicker when it gets hot, and fluid molecules move slower when it turns cold. Usually, the heat process happens more often. This is the Kinetic Molecular Theory.
a theory that the temperature of a body is determined by the average kinetic energy of its particles and that an inflow of heat increases this energy.
Related Questions
burning 12g of urea raise temp of water by 30C what is the enthalpy of combustion for 1kg urea
Answers
The enthalpy of combustion for 1kg of urea is -1223525.84 J/mol.
Urea is a compound that is used in fertilizers and in some plastics.The enthalpy of combustion for urea is the amount of energy that is released when urea is burned. In order to calculate the enthalpy of combustion for 1kg of urea, we need to use the information that is provided to us in the question. Let us start by writing down the balanced equation for the combustion of urea: CO(NH2)2 + 3/2 O2 → CO2 + 2H2O + N2
The balanced equation shows that 1 mole of urea reacts with 1.5 moles of oxygen gas to produce 1 mole of carbon dioxide, 2 moles of water, and 1 mole of nitrogen gas. The enthalpy change for this reaction is equal to the amount of energy that is released when 1 mole of urea is burned.
The heat of combustion (ΔHc) of urea is -632.6 kJ/mol. This means that 632.6 kJ of energy is released when 1 mole of urea is burned. We know that 12g of urea raised the temperature of water by 30°C. We can use this information to calculate the amount of energy that was released when 12g of urea was burned.
The specific heat capacity of water is 4.18 J/g°C. This means that it takes 4.18 J of energy to raise the temperature of 1 gram of water by 1°C. Therefore, it takes 4.18 x 1000 = 4180 J of energy to raise the temperature of 1 kg of water by 1°C.
We know that 12g of urea raised the temperature of water by 30°C. Therefore, the amount of energy that was released when 12g of urea was burned is:
Energy = mass x specific heat capacity x temperature change
Energy = 0.012 kg x 4180 J/kg°C x 30°C
Energy = 1497.6 J
We can now use this information to calculate the enthalpy of combustion for 1kg of urea:
Enthalpy of combustion = energy released / moles of urea burned
Enthalpy of combustion = 1497.6 J / (0.012 kg / 60.06 g/mol)
Enthalpy of combustion = - 1223525.84 J/mol
for such more questions on enthalpy
https://brainly.com/question/14047927
#SPJ8
What heats up faster but loses heat faster?
-Water
-Air
Or
Land
Answers
What volume of 5 M NaOH is needed to create a 100 mL solution of 1 M NaOH?
Answers
Explanation:
MV1=MV2
5×100=1×V
V=500
The volume of 5 M NaOH needed is 20 mL
From the question given above, the following data were obtained:
Molarity of stock solution (M₁) = 5 M
Volume of diluted solution (V₂) = 100 mL
Molarity of diluted solution (M₂) = 1 M
Volume of stock solution (V₁) =?The volume of the stock solution needed can be obtained as follow:
M₁V₁ = M₂V₂5 × V₁ = 1 × 100
5 × V₁ = 100
Divide both side by 5
V₁ = 100 / 5
V₁ = 20 mLTherefore, 20 mL of 5 M NaOH is needed.
Learn more: https://brainly.com/question/24697621
write a balanced chemical equation for the decomposition of asprin
Answers
The balanced chemical equation for the decomposition of aspirin (acetylsalicylic acid) is:
\(2C_{9}H_{8}O_{4} (aspirin) → 2C_{7}H_{6}O_{3} (salicylic acid) + 2CO_{2} (Carbon dioxide) + H_{2}O (water)\)
In this reaction, the aspirin molecule breaks down into salicylic acid, carbon dioxide, and water. The reaction is typically catalyzed by heat or exposure to acidic or basic conditions.
Aspirin, or acetylsalicylic acid, contains ester functional groups that can undergo hydrolysis. Under suitable conditions, the ester bond in aspirin is cleaved, leading to the formation of salicylic acid, which is the primary decomposition product. Additionally, carbon dioxide and water are released as byproducts of the reaction.
The balanced equation shows that for every two molecules of aspirin, two molecules of salicylic acid, two molecules of carbon dioxide, and one molecule of water are formed. Understanding the decomposition of aspirin is important in pharmaceutical and chemical industries to ensure the stability and shelf-life of the compound, as well as to study its breakdown products and potential side reactions.
Know more about aspirin here:
https://brainly.com/question/13533428
#SPJ8
The surface particles of a sample of matter experiences a strong downward pull
and stretch tight enough for a spider to walk on. This is an example of?
HELP PLEASE ITS DUE TODAY, THANK U!
Answers
how to get N-methyl-4-(p-tolyldiazenyl)aniline from benzene and toluene
Answers
The synthesis of N-methyl-4-(p-tolyldiazenyl)aniline can be accomplished in a few steps, as outlined below:
Step 1: Nitration of toluene
Step 2: Reduction of p-nitrotoluene
Step 3: Diazotization of p-toluidine
Step 4: Coupling with N-methylaniline
Toluene is first nitrated to form p-nitrotoluene. This can be done by treating toluene with a mixture of nitric acid and sulfuric acid under controlled conditions. The reaction can be represented as follows:
Toluene + HNO3 → p-nitrotoluene + H2O
The p-nitrotoluene is then reduced to form p-toluidine, using a reducing agent such as iron and hydrochloric acid. The reaction can be represented as follows:
p-nitrotoluene + 6HCl + Fe → p-toluidine + 3H2O + FeCl3
The p-toluidine is then diazotized using nitrous acid to form the diazonium salt. The reaction can be represented as follows:
p-toluidine + HNO2 → p-tolyldiazonium chloride + H2O
The diazonium salt is then coupled with N-methylaniline to form N-methyl-4-(p-tolyldiazenyl)aniline. The reaction can be represented as follows:
p-tolyldiazonium chloride + N-methylaniline → N-methyl-4-(p-tolyldiazenyl)aniline + HCl
Overall reaction:
Toluene + HNO3 → p-nitrotoluene + H2O
p-nitrotoluene + 6HCl + Fe → p-toluidine + 3H2O + FeCl3
p-toluidine + HNO2 → p-tolyldiazonium chloride + H2O
p-tolyldiazonium chloride + N-methylaniline → N-methyl-4-(p-tolyldiazenyl)aniline + HCl
It is important to note that these reactions require careful handling and should only be attempted by individuals with proper training and experience in organic chemistry.
For more question on synthesis click on
https://brainly.com/question/30332351
#SPJ11
1. How isotopes of copper are Cu-63 and Cu-65. How are these two isotopes the same? How are they different?
2. What is an isotope?
3. Why do elements in the same group have similar chemical properties?
4.What are valence electrons?
5.Write the symbol for each of the following elements:
The halogen in period three
The alkali metal in period two
The noble gas in period one
The alkaline earth element in period six
Any transition metal in the 5th period
A metaloid in group 14
A nonmetal in group 16
6. What are two differences between a metal and a nonmeta
PLEASE ASAP!!!!!!!!!!!!!!

Answers
Answer:
question 6. answer is Metals are considered to be electropositive in nature due to their ability to donate electrons. Whereas non-metals are electronegative as they generally accept electrons
Question 13(Multiple Choice Worth 5 points)
(03.05 MC)
Liquid X has a pH of 7 and Liquid Y has a pH of 8.5. Which statement is true?
Liquid X is a base and Liquid Y is an acid.
Liquid X is an acid and Liquid Y is a base.
Liquid X is neutral and Liquid Y is a base.
Liquid X is an acid and Liquid Y is neutral.
Answers
Answer:
Liquid X is neutral and Liquid Y is a base
Explanation:
Acid: pH<7
Neutral: pH=7
Base: pH>7
A driver needs to make a delivery to an office that is 30 km away. The driver has traveled for 45 minutes west down a straight road at 50 km/h. a. Has the driver traveled far enough to reach the office? Support your response. Input Field 1 of 2 Skip to input field
Answers
Answer: Yes
Explanation:
Convert \(30km\) to \(min.\) by using the conversion factor that is given which is \(\frac{50km}{1hr}\).
\(30km/\frac{1hr}{50km}/\frac{60min}{1hr}=36min\)
This means it would take 36 minutes for the driver to reach 30 km
Why is honesty important?
Answers
Honesty leads to a fulfilling, free life. Honesty is not just about telling the truth. It's about being real with yourself and others about who you are, what you want and what you need to live your most authentic life. ... Honesty sharpens our perception and allows us to observe everything around us with clarity.
(brainliest plz)
Calculate the mean free path of electrons in a metal, such as silver, at room temperature form heat capacity and heat conduction measurements. Take EF ¼ 5 eV, K ¼ 4:29 102 J/s m K, and Cel v ¼ 1% of the lattice heat capacity. (Hint: Remember that the heat capacity in (21.8) is given per unit volume!)
Answers
Answer:
= 4 * 10-8 = 400 Angstrom
Explanation:
EF = 5 eV, K = 4.29 x 102 J/(s m K), and Cvel = 1% of the lattice heat capacity
K= 1/3 (Cv)*v*l
v is fermi velocity which is equal to \(v = (2E_f/m)^{0.5}\)
after putting mass of electron as \(9.1 * 10^{-31}kg\) and \(E_f = 5 eV\) we get \(v= 1.33 * 10^6 m/s\)
\(C_v\) is 1% of lattice heat capacity
Heat Capacity of Aluminium is \(0.897 J g^{-1}K^-1\)
Density = \(2.6989 g \ cm^{-3}\)
For lattice heat capacity you need to use the heat capacity for alimunium given and then multiply with density to get per unit volume term
Heat Capacity per unit volume = \(0.897 J g^{-1}K^-1\) * \(2.6989 g \ cm^{-3}\)
\(= 2.42 J K^{-1} cm^{-3} \\\\= 2.42* 10^6 J K^{-1} m^{-3}\)
Cv = 1% of heat capacity per unit volume
\(=0.01 * 2.42* 10^8 J K^{-1} m^{-3} \\\\= 2.42* 10^4 J K^{-1} m^{-3}\)
Putting values in this equation K= 1/3 (Cv)*v*l
\(l = 3K/(C_v * v )\\\\ = 3 * 4.29 * 10^2 / (2.42* 10^4 * 1.33 * 10^6 )\)
\(= 4 * 10^{-8 }\)
= 400 Angstrom
Explain two positive aspects of using methane recapture systems.
Answers
Answer:
Two positive aspects of using methane recapture systems are able to generate significant electricity. Another benefit is that the process of anaerobic digestion creates heat that can be used to warm buildings where animals are kept
Answer: The correct answer is;
Two positive aspects of using methane recapture systems include lowering the impact on greenhouse gasses and the production of energy. Methane is a very potent greenhouse gas that is contributing to global warming. As a result, the recapturing process reduces the methane impacts of global warming by reclaiming and reusing the gas for other purposes. Recaptured methane can be stored and used to generate electricity or used as fuel to power updated vehicles and other engines on the farm. The overall benefits from this combination are reducing impacts causing global warming and lower the cost of electricity or fuel on the farm.
Explanation: This answer has been confirmed correct.
A student predicted the reaction between hydrogen and oxygen gas would yield 125 of water. However, after measuring the water formed in the reaction, student determined that only 110 of water was actually formed. What is the percent yield of this reaction ?
Help
Answers
So in this case the actual yield is 110 and the theoretical yield is 125.
So you would do =(110/125)x100
So the answer would be =88%
Which pictures shows the proper structure of the water molecule? Click the picture to see each choice.


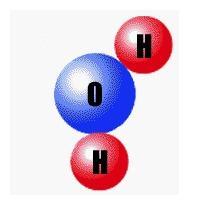

Answers
The third picture is the answer
Explanation:
there are 2 hydrogen atom 1 oxygen
Can someone please help me?

Answers
The IUPAC name for each of the compounds would be:
A. 2,6-Dimethyl octane
B. Octane
What is IUPAC naming?IUPAC naming is a system of naming organic compounds according to the rules set up by the International Union of Pure and Applied Chemistry.
According to these rules:
The longest carbon chain, otherwise known as the parent chain is considered.The parent chain is numbered in such a way that the branching chain or chains (substituents) get the lowest number.The location of each substituent is written. If there is more than one substituent, they are written in alphabetical order.Applying these rules to the structures in the image, the IUPAC names would be as follows:
A. 2,6-Dimethyl octane
B. Octane
More on IUPAC naming can be found here: https://brainly.com/question/16631447
#SPJ1
the monomers of nucleic acids consist of a phosphate group, a five-carbon sugar, and aof blank
Answers
Massive macromolecules called nucleic acids are created from nucleotide monomers. Three things make up a nucleotide: a nitrogenous base, a phosphate group, and pentose sugar (5 carbons).
Nucleic acids, such as DNA and RNA, are what make up an organism's genetic code. Nucleotides, which are composed of distinct subunits, are their monomers. A 5-Carbon sugar (a pentose), a charged phosphate, and a nitrogenous base make up a nucleotide (Adenine, Guanine, Thymine, Cytosine, or Uracil). Nucleotides are the name given to the DNA monomers. Three elements make up a nucleotide: a base, a sugar (deoxyribose), and a phosphate residue. The four bases are thymine (T), adenine (A), cytosine (C), and guanine (G) (T).
To learn more about Nucleotides here,
https://brainly.com/question/29793925
#SPJ4
Select the element(s) that will have ONE unpaired electron in the p orbital.
Ca
N
B
Ar
Br
Answers
Answer: The element B will have ONE unpaired electron in the p orbital.
Explanation:
The electronic configuration of each given element is as follows.
Atomic number of calcium (Ca) is 20.
Ca: \(1s^{2} 2s^{2} 2p^{6} 3s^{2} 3p^{6} 4s^{2}\)
Atomic number of nitrogen (N) is 7.
N: \(1s^{2} 2s^{2} 2p^{3}\)
Atomic number of boron (B) is 5.
B: \(1s^{2} 2s^{2} 2p^{1}\)
Atomic number of argon (Ar) is 18.
Ar: \(1s^{2} 2s^{2} 2p^{6} 3s^{2} 3p^{6}\)
Atomic number of bromine (Br) is 35.
Br: \([Ar] 4s^{2} 3d^{10} 4p^{5}\)
Therefore, boron is the only element that have one unpaired electron in the p-orbital.
Thus, we can conclude that element B will have ONE unpaired electron in the p orbital.
In this short synthetic sequence, provide the organic structures of the missing reactant and the missing product.
Part 1) Draw the missing reagent. Your structure must include a bromine atom.
Part 2) Draw the product, showing stereochemistry.


Answers
The first step of the reaction, we have reaction between a nucleophile and an electrophile.
What is the missing reagent?We can see that in the first step of the reaction, we have reaction between a nucleophile and an electrophile. In this case, the electrophile would have to be an alkyl halide which produces a carbocation as show in the image attached.
What we have here is quite similar or like most of the organic reactions, this reaction occurs in a number of detailed or smaller steps and each step of the reaction is going to help to bring us closer to the end product of the entire steps of the reaction which is wat we target as we carry out the particular reaction.
The second step involves the reduction of the alkyne with the use of a Lindlar catalyst. As such the reaction is poisoned and it stops at the alkyne stage rather than going on to obtain the alkane.
Learn ore about electrophiles:https://brainly.com/question/21773561
#SPJ1

what mass of glucose c6h12o6 would be required to prepare 5000 mL of a 0.215 M solution
Answers
Approximately 194.0 grams of glucose (C6H12O6) would be required to prepare a 5000 mL solution with a concentration of 0.215 M.
To determine the mass of glucose (C6H12O6) required to prepare a 0.215 M solution in 5000 mL, we need to use the formula:
Molarity (M) = moles of solute / volume of solution (in liters)
First, let's convert the volume of the solution from milliliters (mL) to liters (L):
5000 mL = 5000/1000 = 5 L
Now, we can rearrange the formula to solve for moles of solute:
moles of solute = Molarity (M) x volume of solution (L)
moles of solute = 0.215 M x 5 Lmoles of solute = 1.075 mol
Since glucose (C6H12O6) has a molar mass of approximately 180.16 g/mol, we can calculate the mass of glucose using the equation:
mass of solute = moles of solute x molar mass of solute
mass of glucose = 1.075 mol x 180.16 g/mol
mass of glucose = 194.0 g (rounded to three significant figures)
Therefore, approximately 194.0 grams of glucose (C6H12O6) would be required to prepare a 5000 mL solution with a concentration of 0.215 M. It's important to note that the molar mass of glucose used in this calculation may vary slightly depending on the level of precision required.
For more such questions on glucose visit:
https://brainly.com/question/397060
#SPJ8
When fluids are subjected to increases in pressure they tend to do this.
A.) evaporate
B.) contract
C.) expand
D.) solidify
Answers
Answer:
D.) solidify
Explanation:
The average birth weight of domestic cats is about 3 ounces. Assume that the distribution of birth weights is Normal with a standard deviation of 0 4 ounce.
a. Find the birtn weight of cats at the 90th percentile.
b. Find the birth weight of cats at the 10th percentile
Answers
The birth weight of cats at the 90th percentile would be approximately 3.7 ounces. b. The birth weight of cats at the 10th percentile would be approximately 2.3 ounces.
What are the methods of calculating weight?There are three main methods of calculating weight:
1. Balance Beam Scale: A balance beam scale is an example of a mechanical weighing system. It uses a set of calibrated weights to measure the weight of an object.
2. Digital Scale: A digital scale uses an electronic or digital readout to display the weight of an object.
3. Calipers: Calipers are devices used to measure the distance between two points, such as thickness or diameter. They come in various sizes and shapes, depending on the type of measurement the user wants to take.
What is birth weight?Birth weight is the weight of a baby at the time of birth. It is usually measured soon after delivery, with a special baby scale, though sometimes the baby's weight is estimated. The normal range of birth weight is anywhere between 5 lbs 8 oz and 10 lbs, though preterm or premature babies may be significantly lighter. A baby's birth weight is important because it can provide a clue to the baby's overall health. High birth weights may indicate an underlying medical condition, and low birth weights can be a sign of premature or difficult delivery and health risks associated with such a delivery.
To know more about birth weight, visit:
https://brainly.com/question/19262426
#SPJ1
Fill in the [?]:
82 ul = [?]x10^[?] L
![Fill in the [?]:82 ul = [?]x10^[?] L](https://i5t5.c14.e2-1.dev/h-images-qa/contents/attachments/c5wvXAJwcdeECdkhrV3P6zl8SIuUnJSw.png)
Answers
Answer:
8.2×10¯⁵ L
Explanation:
82 μL.
We can convert 82 μL to L by doing the following:
To convert from microlitre (μL) to litre(L), we need to know how many microlitre (μL) that makes up a litre (L)
Recall:
1 μL = 1×10¯⁶ L
Therefore,
82 μL = 82 μL × 1×10¯⁶ L/1 μL
82 μL = 8.2×10¯⁵ L
Therefore, 82 μL is equivalent to 8.2×10¯⁵ L
Mass = 7.2 g Volume = 3 mL Density = ?
Answers
Answer:
2.4
Explanation:
Why is the bond angle in a water molecule less than the bond angle of methane
Answers
Answer:
The H—C—H bond angle in methane is the tetrahedral angle, 109.5°. This angle is obtained when all four pairs of outer electrons repel each other equally. The bond angles in ammonia and in water are less than 109.5° because of the stronger repulsion by their lone pairs of electrons.
Explanation:
hope it helps!
Compare the 13C spectra of starting material and product and explain which peaks can be used to unambiguously determine whether your oxidation was successful. -g
Answers
Answer:
The description of the given question is summarized in the explanation section below.
Explanation:
A maximum of around 82 ppm would be found throughout the beginning material spectrum around CDCl3. This same resulting spectrum maximum of 82 ppm has been lost, as well as the section has a historic high of 215 ppm.
The OH summit at 82 ppm was obvious as well as a significant maximum of around 215 ppm carboxylic acid was observed. That justifies the occurrence of oxidizing, C13 NMR was indeed verified as a reactive organic compound oxidized in carbonyl Ozone.The oxidation state is successful by comparing the 13C spectra of starting material and product.
What is 13C spectra?The 13 carbon spectroscopy also known as 13C NMR is the application of NMR to carbon atom.
A frequent task in synthetic reactions involving rearrangement, conversion, or cyclization is to compare the NMR spectra of the starting material and the result.
Around CDCl3, a high of around 82 ppm can be detected throughout the starting material spectrum.
The section's historic high of 215 ppm has also been destroyed, as has the resulting spectrum maximum of 82 ppm.
The OH peak at 82 ppm was clearly visible, as was a substantial maximum at around 215 ppm carboxylic acid.
Thus, It can say that the oxidation state was successful.
Learn more about 13C spectra, here:
https://brainly.com/question/6427502
For about how long do scientists think wind and solar power can provide us with energy?
Answers
Answer:
about 2-3 years
Explanation:
Because Wind and Solar energy store power and when they store that power it becomes reusable. For example, your house runs out of power in a thunderstorm and the lights go out, what can you mostly rely on, Solar Panels and if it has been cloud all day or all week there might be a chance that you run out of power depending how much power the solar panel has but as long as you don't use much power and the whole week has been sunny, you're fine.
Hope this helps
-Storm
a gas has an initial volume of 3,480 mL and an initial temperature of - 70.0 C. what must be the temperature of the gas in kelvin if its volume is reduced to 2,450 mL
Answers
The temperature of the gas in Kelvin, after its volume is reduced to 2,450 mL, is approximately 143.27 K.
To determine the temperature of the gas in Kelvin after its volume is reduced, we can use the combined gas law, which relates the initial and final conditions of pressure, volume, and temperature for a given amount of gas.
The combined gas law equation is:
(P₁ * V₁) / T₁ = (P₂ * V₂) / T₂
Where P₁ and P₂ are the initial and final pressures, V₁ and V₂ are the initial and final volumes, T₁ is the initial temperature in Kelvin, and T₂ is the final temperature in Kelvin.
Given that the initial volume V₁ is 3,480 mL, the initial temperature T₁ is -70.0 °C (which needs to be converted to Kelvin), and the final volume V₂ is 2,450 mL, we can substitute these values into the equation.
To convert -70.0 °C to Kelvin, we add 273.15 to it, resulting in T₁ = 203.15 K.
Now we can solve for T₂:
(T₂ * V₁) / T₁ = V₂
T₂ = (V₂ * T₁) / V₁ = (2,450 mL * 203.15 K) / 3,480 mL
Simplifying the equation, we find:
T₂ ≈ 143.27 K
Therefore, the temperature of the gas in Kelvin, after its volume is reduced to 2,450 mL, is approximately 143.27 K.
For more question on temperature
https://brainly.com/question/4735135
#SPJ8
Describe the AIR MASSES that would affect the climates of
Florida.
Maine.
Montana.
texas
Answers
The AIR MASSES that would affect the climates of Florida : are maritime tropical air mass and the maritime polar air mass,
Maine : maritime polar air mass and maritime tropical air mass,
Montana : continental polar and maritime polar air masses.
Texas : maritime tropical air masses and continental tropical air masses
What is known as AIR MASSES?An air mass is described as a large body of air with generally uniform temperature and humidity.
An air mass's properties are determined by the region from which it originates. The likelihood that the air mass will take on characteristics of the surface below increases with the amount of time it spends over its source region.
Learn more about air mass at:
https://brainly.com/question/19626802
#SPJ1
Use the scenario to answer the following question. Four scientists observed an area of land that had been cleared of all trees and bushes to make space for a power line. Each scientist had a different argument about the impact clearing this area of land might have on the bird population living in the ecosystem. Which scientist’s argument demonstrates how the ecosystem will MOST LIKELY be impacted? A. Scientist 4: The rate of erosion will decrease causing less food and shelter to be available to the birds. B. Scientist 1: The natural enemies of birds will be eliminated causing less food and shelter to be available to the birds. C. Scientist 2: Weather conditions may change causing less food and shelter to be available to the birds. D. Scientist 3: The population of trees in the environment will decrease causing less food and shelter to be available to the birds.
Answers
Answer:
b
Explanation:t not sure
Answer:
c
Explanation:
Why C is the correct answer? (the question is:Which of the following compounds are NOT aliphatic hydrocarbons?)

Answers
C is the correct option as it contains oxygen along with carbon and hydrogen. Aliphatic hydrocarbons that have had one or more hydrogen atoms .
Aliphatic hydrocarbons that have had one or more hydrogen atoms replaced by a halogen, such as those that have been fluorinated, chlorinated, brominated, or iodized, are known as halogenated aliphatic hydrocarbons.
The group of chemical molecules known as chlorinated aliphatic hydrocarbons is diverse and is characterised by an open-chain structure or a variable number of bonds, which can be single, double, or triple. C is the correct option as it contains oxygen along with carbon and hydrogen.
To know more about Aliphatic hydrocarbon, here:
https://brainly.com/question/30864970
#SPJ1