Answers
Answer:
Its c
Explanation:
i just did it and got it correct
Scientific investigations include a hypothesis that can be shown to be wrong. The correct option is C.
What is Scientific investigation?A scientific investigation is basically a strategy for posing questions and testing potential responses. Observations are usually the starting point for a scientific investigation.
Observations frequently generate questions. Based on scientific knowledge, a hypothesis is a possible logical answer to a scientific question.
The primary goal of a scientific investigation is to increase knowledge. Researchers can discover explanations for natural phenomena and apply their findings to solve real-world problems through observation and experimentation.
The scientific method is an empirical method of acquiring knowledge that has characterized scientific development since at least the 17th century.
It entails careful observation and applying rigorous skepticism to what is observed, given that cognitive assumptions can distort how the observation is interpreted.
Thus, the correct option is C.
For more details regarding Scientific investigation, visit:
https://brainly.com/question/8386821
#SPJ2
Related Questions
a polygon with four equal sides ands angle
Answers
A rhombus is a parallelogram with all four sides congruent to each other. diamond-like shape. A square is a parallelogram with four congruent sides and four right angles. In other words, a square is a rectangle and a rhombus.
how boyle's temperature is related to Vander waal's constants a and b?
Answers
The ratio of Boyle's temperature and critical temperature is 278. both a and b are present in Boyle's temperature.
What is boyles temperature ?Boyle temperature can be defined as the point in the temperature range in which a real gas starts to behave like an ideal gas at a pressure range.
The temperature at which the second coefficient in the expression becomes zero is also known as a Boyle temperature.
In Boyle's Temperature , TB=aRb.
Critical Temperature , TC=8a27Rb.
a and b are van der Waal's constants. R is molar gas constant. The Ratio of both temperatures
= TB/TC = aRb/8a27Rb
Both the critical temperature and boyles temperature have the Vander Waal's constant 'a' and 'b'.
learn more about boyles temperature from
https://brainly.com/question/26040104
#SPJ1
ANSWER QUICK PLEASE AND THANK YOU

Answers
One oxygen atom shares two electron with two hydrogen atoms in this way water molecules are formed. The bond between oxygen and hydrogen is covalent bond.
What is covalent bond ?An electron exchange that results in the formation of electron pairs between atoms is known as a covalent bond. Bonding pairs or sharing pairs are the names given to these electron pairs.
Covalent bonding is the stable equilibrium of the attractive and repulsive forces between atoms when they share electrons.
According to the amount of shared electron pairs, there are three different forms of covalent bonds. single covalent bond is one type of covalent bond. covalent double bond and covalent triple bond.
Thus, option B is correct .
To learn more about covalent bond follow the link ?
https://brainly.com/question/10777799
#SPJ1
(Gamergirl223)
A scientist is studying the liquid shown here. She thinks the liquid is a mixture.
Describe an investigation she could do to demonstrate that the liquid is in fact a
combination of substances

Answers
The answer is: To demonstrate that the liquid is a mixture, the scientist can perform the chromatography.
Mixture: They are formed when two elements or compounds are combined physically and not chemically.
What are the types of mixtures?
Mixtures are divided into two categories :Homogeneous and heterogeneous.Homogeneous mixtures- They have the same composition throughout their mass and there are no visible boundaries between the components of the mixture. Example- Sugar solution, salt solution, alloys, alcohol dissolved in water.Heterogeneous mixture- They do not have the same composition throughout there are visible boundaries between the components of the mixture. Example- Milk, sand, and iron fillings, solution of \(CaCO_3\), solution of \(K_2Cr_2O_7\).To demonstrate the the given liquid is a mixture, the scientist can perform Chromatography.Chromatography is based on the different rates of adsorption of components of a mixture on a suitable adsorbent.When a drop of a mixture of substances is put on a chromatogram paper and the paper is dipped in a solvent, the substance that is more soluble in the solvent rises faster compared to others. And due to this difference in adsorption of different substances, they are separated by the method of chromatography. If two spots are seen on a chromatogram that means the liquid is a mixture and if only one spot is visible, that suggests that liquid is a pure liquid.To learn more about the mixture and chromatography, visit:
https://brainly.com/question/1394204
#SPJ9
According to the Clausius theorem, the cyclic integral of
for a reversible cycle is zero.
Answers
Answer:
Yes it is true
Explanation:
This is because the Clausius theorem states that The cyclic integral always has two defined results. The results include it being less than or equal to zero under certain conditions.
When the system consists of only reversible processes, the cyclic integral is equal to zero. If it consists of and irreversible processes, the integral is usually less than zero.
WILL MAKE BRAINLIEST!! Show steps!
1.) A chemist needs to prepare 4.00 L of a 0.50 M solution of potassium permanganate (KMnO4). What mass of KMnO4 does she need to make the solution? This problem is similar to Sample Problem 16.3.
Show your work (4 Points) (Answer: 316.08 g)
2.) What volume of a 1.50 M solution of KCl needs to be diluted in order to prepare 2.40 L of a 0.0750 M solution? This problem is similar to Sample Problem 16.4. Show your work. (4Points) (Answer: 0.12 L)
3.) Determine the molality of a solution prepared by dissolving 20 g of sodium chloride (NaCl) in 150. g of water. This problem is similar to Sample Problem 16.5. Show your work. (4 Points) (Answer: 2.3 mol/Kg)
Answers
1.) Chemist needs 79.02 g of KMnO4 to make 4.00 L of a 0.50 M solution. 2.) You will need to use 0.12 L of 1.50 M solution to make 2.40 L of 0.0750 M solution. 3.) Solution has a molality of 0.00229 mol/kg.
What is molality?Molality is defined as a measure of number of moles of solute in a solution corresponding to 1 kg or 1000 g of solvent.
1.) As, mass = moles x molar mass
Molar mass of KMnO4 is 158.03 g/mol
So, mass = 0.50 mol x 158.03 g/mol = 79.02 g
Therefore, the chemist needs 79.02 g of KMnO4 to make 4.00 L of a 0.50 M solution.
2.)As , C1V1 = C2V2
Here C1 is initial concentration, V1 is initial volume, C2 is final concentration and V2 is the final volume.
1.50 M x V1 = 0.0750 M x 2.40 L
Now, V1 = (0.0750 M x 2.40 L) / 1.50 M
So, V1 = 0.12 L
You will need to use 0.12 L of the 1.50 M solution to make 2.40 L of the 0.0750 M solution.
3.) Formula for molality is: m = (moles of solute) / (kg of solvent)
As, moles = (mass of solute) / (molar mass of solute)
= (20 g) / (58.44 g/mol) = 0.344 mol
m = (0.344 mol) / (150 g / 1000 g/kg) = 0.00229 mol/kg
So, the solution has a molality of 0.00229 mol/kg.
To know more about molality, refer
https://brainly.com/question/1370684
#SPJ1
What does friction do? -Getting your stats up-
Answers
Part C
Place the copper strips in test tubes 3 and 6. If the strips are too wide, you may bend them to fit them in the test tubes.
Place the magnesium strips in test tubes 1 and 4. If necessary, use the stirring rod to push the strips down until they’re entirely submerged.
Place the zinc strips in test tubes 2 and 5.
Record your observations of the appearance of the substances in the test tubes immediately after adding the metal strips. Include any evidence of a chemical reaction. If there’s no evidence of a reaction, write “no reaction.”
*What best goes in the blanks?*

Answers
Based on the reactivity of the metals:
No reaction occurs for the copper strips in test tubes 3 and 6. Hydrogen is displaced by the magnesium strips in test tubes 1 and 4. The reaction more vigorous in the acid of higher concentration.Hydrogen is displaced by the zinc strips in test tubes 2 and 5. The reaction more vigorous in the acid of higher concentration.What occurs when the metals copper, magnesium and zinc are placed in test tubes containing hydrochloric acid?
The ability of metals to displace hydrogen from dilute acids depends on the reactivity of the metal.
Reactive metals such as zinc and magnesium which occur higher than hydrogen in the activity series of metals will displace hydrogen from dilute acids such as dilute hydrochloric acid.
Copper cannot displace hydrogen from acids because it is less reactive than hydrogen.
The rate of reaction increases with increase in concentration of the acid.
Therefore, no reaction occurs for the copper strips in test tubes 3 and 6.
Hydrogen is displaced by the magnesium strips in test tubes 1 and 4, with the reaction more vigorous in the acid of higher concentration.
Hydrogen is displaced by the zinc strips in test tubes 2 and 5, with the reaction more vigorous in the acid of higher concentration.
In conclusion, reactive metals such as magnesium and zinc displace hydrogen from acids while a less reactive metal such as copper do not.
Learn more about reactive metals at: https://brainly.com/question/24753013
#SPJ1
List 4 significant problems with nuclear power plants.xx
Answers
Answer:
Cost.
Weapons Proliferation Risk.
Meltdown Risk.
Mining Lung Cancer Risk.
Explanation:
Hope this helped!!!
Thermal energy depends on what two things?
Answers
Answer:
temperature and mass
Explanation:
porque
Hope this helps :)
Convert 100.6 Kelvin to degrees C.
°C = K - 273
[?] °C
Answers
Answer:
-172.6 °C
Explanation:
You want to know the Celsius equivalent of the temperature 100.6 K.
ConversionThe relation is ...
C = K - 273.15
C = 100.6 -273.15 = -172.55
The temperature is -172.55 °C, about -172.6 °C.
__
Additional comment
We have rounded to tenths, because that is precision of the temperature given. If you use 273 as the conversion constant, you will get -172.4.
A 0.552-g sample of ascorbic acid (vitamin C) was dissolved in water to a total volume of 20.0 mLand titrated with 0.1103 MKOH, and the equivalence point occurred at 28.42 mL. The pHof the solution at 10.0 mL of added base was 3.72.
Answers
The molar mass is 176.1 g/mol , and Ka for vitamin C is 1.03 x 10⁻⁴
The equation for the reaction is as shown in the attached diagram below
the reaction of Sodium Hydroxide, NaOH with ascorbic acid, C₆H₈O₆ is similar to the reaction of Potassium Hydroxide, KOH with ascorbic acid as show
C₆H₈O₆ + KOH ⇒ C₆H₇O₅K + H₂O
to produce one mole of potassium ascorbate and water
now,
0.1103 M KOH is contained in 1000mL
x moles is contained in 20mL
cross multiply making x the subject
No of moles of KOH = (0.1103 x 28.42)/1000 = 0.003135 moles
or
Moles of KOH = 0.1103 x 0.02842 L = 0.003135 = moles ascorbic acid
Molar mass = 0.552 g / 0.003135 mol = 176.1 g/mol
Moles of ascorbic acid = 0.552 / 176.1 =0.00313
moles NaOH = 0.0100 L x 0.1103 =0.001103
C₆H₈O₆ + OH⁻ >> C₆H₇O₆⁻ + H2O
Moles ascorbic acid in excess = 0.00313 - 0.001103 = 0.002027
Moles C₆H₇O₆⁻ = 0.001103
total volume = 20 + 10 = 30 mL = 0.030 L
concentration ascorbic acid = 0.002027 / 0.030 =0.0676 M
concentration C₆H₇O₆⁻ = 0.001103 / 0.030 =0.0368 M
pH = pKa + log [C₆H₇O₆⁻] / [C₆H₈O₆]
3.72 = pKa + log 0.0368 / 0.0676
3.72 = pKa - 0.264
pKa =3.984
Ka = 10^-3.984
=1.03 x 10⁻⁴
Your question is incomplete most probably your full question was
A 0.552-g sample of ascorbic acid was dissolved in water to a total volume of 0.20 mL and titrated with 0.1103 M KOH. The equivalence point occurred at 28.42 mL. The pH of the solution at 10.0mL of added base was 3.72. From this data, determine the molar rmass and Ka for vitamin C.
To look more about molar mass click here
brainly.com/question/12127540
#SPJ4

How many moles of aluminum are needed to react completely with 2 moles FeO?
Al(s)+3FeO(s)—>3Fe(s)+Al2O3(s)
Answers
0.66 moles of aluminum are needed to react completely with 2 moles of FeO.
Given,
the chemical equation is,
Al(s)+3FeO(s)—>3Fe(s)+Al2O3(s)
1 mole of aluminum is needed to react completely with 3 moles of FeO.
then,
no of moles of aluminum are needed to react completely with 2 moles of FeO = 2/3 = 0.66 moles
Hence, 0.66 moles of aluminum are needed to react completely with 2 moles of FeO.
What is aluminum?A chemical element with the symbol Al and atomic number 13 is known as aluminium (or aluminium in American and Canadian English). Aluminum has a density that is around one third that of steel, which is lower than that of most common metals. It has a strong affinity for oxygen, and when exposed to air, creates a protective oxide coating on the surface. Aluminum visually resembles silver due to similarities in colour and light-reflecting properties. It is ductile, soft, and non-magnetic. Aluminum is the twelfth most prevalent element in the universe based on the frequency of its one stable isotope, 27Al, which is also the only stable isotope. Radiodating makes use of 26Al's radioactivity. A chemical element with the symbol Al and atomic number 18 is called aluminium (aluminium in American and Canadian English).
Learn more about aluminum here :
brainly.com/question/246454
#SPJ13
Iron reacts with chlorine to form iron(III) chloride.
2Fe + 3Cl2 → 2FeCl3
What mass (in grams) of chlorine gas is needed to react with 251 grams of iron?
Select one:
a.
71 grams
b.
392 grams
c.
479 grams
d.
622 grams
Answers
The mass (in grams) of chlorine gas is needed to react with 251 grams of iron is 479 grams. Option C.
To determine the mass of chlorine gas needed to react with 251 grams of iron, we need to use the stoichiometry of the balanced chemical equation:
2Fe + 3Cl2 → 2FeCl3
From the balanced equation, we can see that 2 moles of iron (Fe) react with 3 moles of chlorine gas (Cl2) to produce 2 moles of iron(III) chloride (FeCl3).
To calculate the mass of chlorine gas, we can follow these steps:
Step 1: Convert the given mass of iron (Fe) to moles.
Using the molar mass of iron (Fe), which is approximately 55.85 g/mol, we can calculate the number of moles of iron:
moles of Fe = mass of Fe / molar mass of Fe
moles of Fe = 251 g / 55.85 g/mol
moles of Fe ≈ 4.5 mol (rounded to one decimal place)
Step 2: Use the mole ratio from the balanced equation to find the moles of chlorine gas (Cl2) needed.
From the balanced equation, we know that 2 moles of Fe react with 3 moles of Cl2. Therefore, the moles of Cl2 can be calculated as:
moles of Cl2 = (moles of Fe / 2) * 3
moles of Cl2 = (4.5 mol / 2) * 3
moles of Cl2 ≈ 6.75 mol (rounded to two decimal places)
Step 3: Convert the moles of chlorine gas to grams.
Using the molar mass of chlorine gas (Cl2), which is approximately 70.90 g/mol, we can calculate the mass of chlorine gas:
mass of Cl2 = moles of Cl2 * molar mass of Cl2
mass of Cl2 = 6.75 mol * 70.90 g/mol
mass of Cl2 ≈ 479 grams (rounded to the nearest whole number) Option C is correct.
For more such question on mass. visit :
https://brainly.com/question/19385703
#SPJ8
What is the number 0.0513 expressed in scientific notation?
Answers
Answer:
5.13x10>-2
Explanation:
from a gas to a liquid
from a liquid to a gas
from a solid to a liquid
from a gas to a plasma
Answers
Answer:Condensation
Evaporation
melting
ionization
Explanation:
Hope this helps :)
From a gas to a liquid: This is called condensation ; From a liquid to a gas: This is called evaporation or vaporization ; From a solid to a liquid: This is called melting ; From a gas to a plasma: This is called ionization.
What is condensation?Process by which water vapor in air is changed into liquid water is called condensation.
From a gas to a liquid: This is called condensation. It occurs when gas loses heat energy and particles start to slow down and move closer together, eventually forming liquid.
From liquid to a gas: This is called evaporation or vaporization. It occurs when liquid gains enough energy to break the intermolecular forces between the particles, causing them to become gas.
From a solid to liquid: This is called melting. It occurs when solid absorbs enough energy to overcome the intermolecular forces holding its particles together, causing particles to move more rapidly and become liquid.
From a gas to a plasma: This is called ionization. It occurs when gas is subjected to strong electric field, heat, or radiation and some or all of atoms or molecules lose their electrons, creating positively charged ions and negatively charged electrons. This mixture of charged particles is called plasma.
To know more about condensation, refer
https://brainly.com/question/1268537
#SPJ1
empirical formula for H2Cl2O6
Answers
Answer:
HClO₃
Explanation:
Empirical formula of a chemical compound is defined as the simplest ratio of atoms presents in the compound (In whole numbers).
For the H₂Cl₂O₆ the empirical formula could be obtained dividing in 2 the formula. That is:
HClO₃ → This is the simplest ratio of atoms presents in the compound, that is, the empirical formula
what is neutron number for calcium
Answers
Answer:
20 neutrons
Explanation:
(not really any just look at a periodic table)
What happens to the amount of solution when we add food colour to it?
Answers
Answer:
We need more? What else is in the question? This is unanswerable.
Explanation:
Which of the following is true about the differences between the "Plum Pudding" model and Rutherford's model? There are two possible answers.
A. Both models describe food.
B. The plum pudding model had no deflection of particles because it lacked a nucleus
C. Rutherford's model showed deflection of particles because the nucleus has positive and neutral particles.
D. The plum pudding model and Rutherford's model are the same
Answers
5. How many moles are present in 4.20x10^24 atoms of Pb
Answers
Explanation:
\(57816 \: moles\)
are present in 4.20x10^24 atoms of Pb
Answer:
7 moles
Explanation:
(4.2*10^24)/(6*10^23)=7
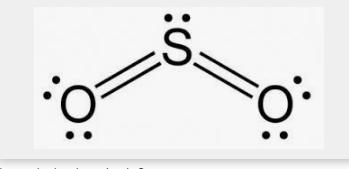
can you draw so2 lewis strucure i sent a picture what i did and u can explain if its wrong

Answers
Answer
The Lewis structure of SO2 (stable form) is shown below;
The unstable form SO2 Lewis structure is also shown below:

C. At an old gas station, gasoline leaks from an underground storage tank.
Classification:
Explanation:
Answers
To pevent gasoline leaks, regular maintenance checks and replacement of damaged underground tanks is required.
What causes gasoline leak?Gasoline leaks occurs when tanks are rusty and corroded or where there are loose fitting of pipes and connection points.
Gasoline leak presents a serious environmental hazard as well as threat to life and properties.
Gasoline leaks can cause fire outbreaks which would destroy properties and endanger lives.
Gasoline leaks can also cause land and water pollution killing important aquatic and soil organisms.
Therefore, regular maintenance checks and replacement of damaged underground tanks will prevent gasoline leaks.
Learn more about gasoline leaks at: https://brainly.com/question/10338424
Answer:
To pevent gasoline leaks, regular maintenance checks and replacement of damaged underground tanks is required.
What causes gasoline leak?
Gasoline leaks occurs when tanks are rusty and corroded or where there are loose fitting of pipes and connection points.
Gasoline leak presents a serious environmental hazard as well as threat to life and properties.
Gasoline leaks can cause fire outbreaks which would destroy properties and endanger lives.
Gasoline leaks can also cause land and water pollution killing important aquatic and soil organisms.
Therefore, regular maintenance checks and replacement of damaged underground tanks will prevent gasoline leaks.
Explanation:
Give the person who answered this question for real brainliest, they deserve it :D
A sample of chlorine gas starting at 688 mm Hg is placed under a pressure of 994 mm Hg and reduced to a volume of 500.2 mL. What was the initial volume, in mL, of the chlorine gas container if the process was performed at constant temperature?
Answers
(P1V1=nRT1)/(P2V2=nRT2)
Constant temperature → (P1V1)/(P2V2)
688 mm Hg → 0.905 atm
994 mm Hg → 1.30789 atm
500.2 mL → 0.5002 L
(P1V1)/(P2V2)
(0.905)V1/(1.30789)(0.5002)
V1 = 0.72288 L → 722.88 mL
Place the following in order of increasing entropy at 298 K.
Ne Xe He Ar Kr
A) He < Kr < Ne < Ar < Xe.
B) Xe < Kr < Ar < Ne < He.
C) Ar < He < Ar < Ne < Kr.
D) Ar < Ne < Xe < Kr < He.
E) He < Ne < Ar < Kr < Xe.
Answers
Answer:
E
Explanation:
The simple rule of thumb is that as the atomic or molar mass of an atom increases, the entropy increases. This means that more the mass more will be entropy.
The atomic mass of various elements is as follows
Ne = 20 g/mol
Xe = 131 g/mol
He = 4 g/mol
Ar = 40 g/mol
Kr = 84 g/mol
Therefore, the order of increasing entropy must be
He<Ne<Ar<Kr<Xe.
Hence, option E is the correct answer.
b. How many kJ of heat are needed to completely vaporize 50.0g of water at 100°C? [Ans:113. kJ]
Answers
The amount, in kJ, of heat needed to completely vaporize 50.0g of water at 100°C is 118.8 kJ.
Heat of vaporization of waterThe heat needed to completely vaporize 50.0g of water at 100°C can be calculated using the following formula:
q = m x Hv
where:
q is the heat needed in joules (J)m is the mass of water in grams (g)Hv is the heat of vaporization of water which is approximately 40.65 kJ/mol at standard temperature and pressure.First, we need to convert 50.0g to moles by dividing by the molar mass of water which is approximately 18.015 g/mol3:
moles of water = 50.0 g / 18.015 g/mol moles of water = 2.776 mol
Thus:
q = (2.776 mol) x (40.65 kJ/mol) q = 112.8 kJ
In other words, 112.8 kJ of heat is needed to completely vaporize 50.0g of water at 100°C.
More on heat of vaporization can be found here: https://brainly.com/question/12625048
#SPJ1
Which of the arrows in the energy-level diagram represent transitions involving the greatest change in energy?

Answers
The transition that involves the greatest change in energy is the transition shown by (f)
What is energy transition in the atom?When an electron moves from one energy level to another, this is referred to as an energy transition in an atom. An atom's electrons can be found in various orbitals or energy levels, and because these levels are quantized, only specific values are permitted.
The transition in f involves a movement from n = 1 to n =4 that shows a great involvement of energy for such to occur.
Learn more about energy transition:https://brainly.com/question/29376664
#SPJ1
Convert 8.876 × 10^12 m^2 to units of km^2.
Answers
Answer:
\(8.876\times 10^{18}\ km^2\)
Explanation:
In this problem, we need to convert \(8.876 \times 10^{12}\ m^2\) to km².
We know that,
1 km = 1000 m
⇒ 1 km² = 10⁶ m²
So,
\(8.876 \times 10^{12}\ m^2=8.876 \times 10^{12}\times 10^6\ km^2\\\\=8.876\times 10^{18}\ km^2\)
So, \(8.876 \times 10^{12}\ m^2\) is equal to \(8.876\times 10^{18}\ km^2\).
Which of the following solutions would have the highest pH? Assume that they are all 0.10 M in acid at 25°C. The acid is followed by its Ka value.
a. HCHO2, 1.8 x 10-4
b. HF, 3.5 x 10-4
c. HClO2, 1.1 x 10-2
d. HCN, 4.9 x 10-10
e. HNO2, 4.6 x 10-4
Answers
Answer:
\(HCN~~Ka=4.9x10^-^1^0\)
Explanation:
In this case, we have to remember the relationship between the Ka value and the pH. We can use the general reaction for any acid with his Ka value expression:
\(HA~->~H^+~+~A^-\) \(Ka=\frac{[H^+][A^-]}{[HA]}\)
In the Ka expression, we have a proportional relationship between Ka and the concentration of \(H^+\). Therefore, if we have a higher Ka value we will have a smaller pH (lets keep in mind that with a higher
So, if we have to find the higher pH value we need to search the smaller Ka value in this case \(HCN~~Ka=4.9x10^-^1^0\).
I hope helps!
HCN has the highest pH among all the acids listed in the question.
The Ka is called the acid dissociation constant. It shows the extent to which an acid is ionized in water. The pH shows the hydrogen ion concentration of water. The higher the Ka, the higher the hydrogen ion concentration and the lower the pH.
Hence, HCN has the lowest Ka and the lowest hydrogen ion concentration. Therefore, HCN has the highest pH among all the acids listed in the question.
Learn more: https://brainly.com/question/6505878
The following are reactants in the chemical reaction shown below.
6CO2 + 6H2O --> C6H12O6 + 6O2
A. CO2 and H2O
B. C6H12O6 and O2
C. H2O and O2
D. CO2 and C6H12O6
Answers
Answer:
A
Explanation:
Reactants are on the right side of the chemical reaction