Answers
Option B. The intermolecular forces present in C4H10 are Dispersion forces and dipole-dipole forces.
What is intermolecular force?
Intermolecular forces are the forces that hold two or more molecules together, creating a compound or a substance.
There are four types of intermolecular forces :
ion-ion forces, ion-dipole forces, dipole-dipole forces, and dispersion forces, depending on the molecules involved. Each type of intermolecular force depends on the physical properties of the molecules involved in the reaction.
C4H10, also known as butane, is a chemical compound with four carbon atoms and ten hydrogen atoms. It is an alkane that is usually in gas form at room temperature and pressure.
Because it contains only nonpolar covalent bonds, butane molecules lack a permanent dipole moment, and dipole-dipole forces do not exist between them. Dispersion forces, on the other hand, do exist between butane molecules due to instantaneous dipoles. Dispersion forces occur as a result of electron movement, which can create a temporary imbalance of electrons in one molecule that induces another molecule's electrons to be attracted.
The dispersion forces in butane are weak because it is a nonpolar molecule.Therefore, the intermolecular forces present in C4H10 are Dispersion forces and dipole-dipole forces.
For similar question on molicular breaker
https://brainly.com/question/31130413
#SPJ11
The intermolecular forces present in \(C_4H_{10}\) are dispersion forces only. Correct option is a.
When the charge distribution of a molecule is averaged across time, it is spherically uniform it is called a nonpolar molecule.
\(C_4H_{10}\) (butane) is a nonpolar molecule, so it does not have any dipole-dipole forces or hydrogen bonding.
Dispersion forces are described as a transient attractive force resulting from the transient dipole production in a nonpolar molecule.
The positive end of one polar molecule and the negative end of another polar molecule are attracted to one another by dipole-dipole forces.
The only intermolecular force present is dispersion forces, which are weak attractive forces between temporary dipoles in the molecules.
Hence option a. Dispersion forces only is correct.
For similar question on butane.
https://brainly.com/question/26954028
#SPJ11
Related Questions
which statements describe what happen in a redox reaction?
a. one atom gains electrons and one loses electrons
b. the cations on two substances trade places
c. electrons move from one substance to another
d. there are two oxidation reactions
Answers
Answer:
A or C
Explanation:
I don't know if I'm correct but I think it's A or C you don't have to choose them.
pleaseeeee helpppp!!!!!!

Answers
Answer:
90% of people marry there 7th grade love. since u have read this, u will be told good news tonight. if u don't pass this on nine comments your worst week starts now this isn't fake. apparently if u copy and paste this on ten comments in the next ten minutes you will have the best day of your life tomorrow. you will either get kissed or asked out in the next 53 minutes someone will say i love u
A rock dropped in a graduated cylinder raises the level of water from 20 mL to 35 mL. The rock has a mass of 45 g. What is the density of the rock?
Answers
Draw structures for compounds that meet the following descriptions in proton NMR:a) C2H6O; one singletb) C3H7Cl; one doublet and one septetc) C4H8Cl2O; two tripletsd) C4H8O2; one singlet, one triplet, and one quartet
Answers
Answer:
The structures are shown in the attachments.
Explanation:
Please see the attachments below for the structures.
Explanation:
For the structure in (a)There is only one environment denoted as 'a'.
Using the (n+1) rule to determine the multiplicity, m
where is the number of neighboring Hydrogen, then
Since, n = 0, then
m = 0 + 1 = 1 (which means singlet)
Hence, one singlet
For the structure in (b)There are two environments denoted as 'a' and 'b'
For 'a' ; n = 6
Hence, m = n + 1
m = 6 + 1 = 7 (this means septet)
For 'b'; n = 1
then, m = n + 1
m = 1 + 1 = 2 (this means doublet)
Hence, one septet, one doublet
For the structure in (c)There are two environments denoted as 'a' and 'b'
For 'a' ; n = 2
then, m = n + 1
m = 2 + 1 = 3 (this means triplet)
For 'b' ; n = 2
then, m = n + 1
m = 2 + 1 = 3 (this means triplet)
Hence, two triplets
For the structure in (d)There are three environments denoted as 'a', 'b', and 'c'
For 'a' ; n = 0
Hence, m = n + 1
m = 0 + 1 = 1 (this means singlet)
For 'b' ; n = 3
then, m = n + 1
m = 3 + 1 = 4 (this means quartet)
For 'c' ; n = 2
then, m = n + 1
m = 2 + 1 = 3 (this means triplet)
Hence, one singlet, one triplet, and one quartet


what is the use of hexane in contact cement?
Answers
The main use of hexane is as a solvent to extract edible oils from seed and vegetable crops. Hexane are used as solvents for contact cement, varnishes, and inks.
What is hexane used for ?Hexanes are mostly used as a solvent. It is used to extract edible oils from seeds and vegetables and as a cleaning agent. The high levels of hexane causes mild central nervous system effects it includes dizziness, slight nausea, and headache etc.
In the construction industry, the hexane is an effective chemical which is use as the strongest organic solvents. It dissolves chemical material which allows the objects to bind together to form a strong force.
Thus, Hexane are used as solvents for contact cement, varnishes, and inks.
To learn more about the hexane, follow the link;
https://brainly.com/question/13258065
#SPJ1
A gas is in a sealed container. How do you think the pressure will change if the container is cooled. explain your answer
Answers
what three things might influence a slide when conditions are right?
Answers
Answer:
3
Explanation:
The three things that can affect a condition to enforce a slide is friction, weight, and slope of an area. Solutions that can make flow easily are those that can create less friction depending on the condition of an environment where it is present.
A student, adds 136.92 g of sucrose (FW = 342.31) to exactly 400.0 mL of dH2O. The total solution volume increased to 420.0 mL after the sucrose was dissolved and the solution was thoroughly mixed. What is the molarity of the final solution?
Answers
The molarity of the final solution is : 0.953 M
Given data :
mass of sucrose = 136.92 g
volume of solvent= 400ml
Total volume of solution = 420 ml
First step : Determine moles of sucroseMoles of sucrose = mass / molar mass
= 136.92 / 342.31
= 0.40 mol
molarity of final solution = 0.40 / 0.420 = 0.953 M.
Hence we can conclude that The molarity of the final solution is : 0.953 M
Learn more about molarity : https://brainly.com/question/17138838
Which two structures are connected by the ear bones?
eardrum and stirrup
stirrup and auditory nerve
auditory nerve and cochlea
cochlea and eardrum
Answers
Answer:
I THINK it is A
Explanation:
Answer: it is the eardrum caus ei used it on my test and as well got it right
Explanation: because it just is
\(hut7utpojghuire\\kjionbenhrmhbnhknokgn\\bihkjihjgnhrk\\\\gnthnrt\\hgnth\\\alpha thtrnhjn4t also this is for fun to me\)
People living in some countries have a difficult time obtaining foods that are high in vitamin A. Which advantage of genetic technologies would these people most benefit from? O higher tolerance of crops to temperature changes O decreased crop resistance to disease O lowered cost of food production O increased nutritional value of crops hurry up asap
Answers
Answer:
the answer i believe is increased nutritional value of crops
Explanation:
Answer: increased nutritional value of crops
Explanation:) edge 2022
If anyone could help I’d appreciate it

Answers
Answer: 2.00 mol
Explanation:
The balanced chemical equation is:
\(2H_2(g)+O_2(g)\rightarrow 2H_2O(g)\)
According to stoichiometry :
2.00 moles of \(H_2\) require = 1.00 moles of \(O_2\)
Thus 2.00 moles of \(H_2\) will require=\(\frac{1}{2}\times 2.00=1.00moles\) of \(O_2\)
Thus both will act as limiting reagents and will be fully consumed.
2.00 moles of \(H_2\) will form = 2 moles of \(H_2O\)
Thus 2.00 moles of \(H_2\) will form = \(\frac{2}{2}\times 2.00=2.00moles\) of \(H_2O\)
Thus 2.00 moles of \(H_2O\) will be produced from the given masses of both reactants.
If a gas effuses 2.17 times faster than Xe, what is its molar mass?
Answers
Answer:
Molar mass = 27.88 g/mol
Explanation:
The relationship between how gases effuses is given by Graham's law of effusion. This law is given as;
Rg / Rxe = \(\sqrt{}\)(Mxe / Mg)
Where;
Rg = Rate of effusion of gas g
RXe = Rate of effusion of Xe
Mxe = Molar mass of Xe = 131.29 g/mol
Mg = Molar mass of gas g
From the question;
Rg : Rxe = 2.17 : 1
Rg / Rxe = 2.17 / 1 = 2.17
2.17 = \(\sqrt{}\)131.29 / Mg
Squaring both sides
4.7089 = 131.29 / Mg
Mg = 131.29 / 4.7089
Mg = 27.88 g/mol
Which statement best describes an O3 molecule?
► View Available Hint(s)
Answers
What is the percent yield for the reaction below when 364 g SO2 and 42.0 g
02 produce 408 g SO3?
2SO₂(g) + O₂(g) → 2SO3(g)
A. 89.7%
B. 97.1%
C. 51.5%
D. 100%
Answers
C. 51.5% is the percent yield for the reaction below when 364 g SO2 and 42.0 g O2 produce 408 g SO3
How is percentage yield calculated?The actual yield is determined by calculating the quantity of the product created. We can estimate the percentage yield by dividing the actual yield by the theoretical value. The percentage yield is the difference between the quantity of product that was actually created and the maximum calculated yield.
2SO₂(g) + O₂(g) → 2SO3(g)
SO₂ mass => 364g
O₂ mass => 42g
SO3 mass => 408g
Theoretical yield of SO3:
mole of O₂ => 42g/32g => 1.31
according to the chemical equation:
1 mole of oxygen => 2 moles of SO3
1.31 mole O2 => x moles of SO3
x=> 2 x 1.31 => 2.62
Hence, the mass of SO3 => 2.62 x 80.06 => 209.75g
The percentage of yield => Actual yield / theoretical yield x 100
=> 209.75/408 x 100 => 51.45 => 51.5%
What differs the theoretical yield from the actual yield?The theoretical yield is the yield that is derived using a balanced chemical reaction. What you actually acquire from a chemical reaction is the actual yield.
Learn more about percentage yield here:
brainly.com/question/29767432
#SPJ1
Nerve impulses are picked up by a neuron’s:
(1) dendrites
(2) axon
(3) synapse
(4) neurotransmitter
Answers
Please help will give brainliest to right answer!!
You add 1.5 moles of HF to 6 liters of water. The concentration is at equilibrium when [H+] is at 0.10 M. What is the Ka of HF? HF -> H+ + F-.
A) 0.067
B) 0.10
C) 0.25
D) 1.5
Answers
Answer:
C
Explanation:
NP
What periodic trend does the atomic radius follow? A. It increases from left to right. B. It decreases from top to bottom. C. It stays the same across the table. D. It decreases from left to right.
Answers
Answer: D
Explanation:
It increases from right to left and down the periodic table. So your answ3er would be D. that it is decreasing from left to right.
Select the TWO statements that are true about the amount and types of energy that are visible when each of the light bulbs are shining.
A. The incandescent light bulb does not create as much as much light energy as the fluorescent light bulb.
B. When the fluorescent light bulb is lit, more light energy is visible than thermal energy.
C. The thermal energy is stored in the incandescent light bulb before it is transformed into light energy.
D. More thermal energy is visible when the fluorescent light bulb is lit.

Answers
A. The incandescent light bulb does not create as much as much light energy as the fluorescent light bulb.
Why do you think fluorescent bulbs are more energy-efficient than incandescent ones?Incandescent light bulbs have the drawback of wasting a lot of electricity due to heat. All the energy used to create heat is a waste because heat does not produce light, and the light bulb's intended function is to produce light. Thus, incandescent lamps are quite ineffective.
Why does a fluorescent tube produce light that is brighter and uses less energy than an incandescent lamp?Mercury vapor is excited by an electric current in the gas to produce short-wave ultraviolet light, which illuminates a phosphor coating inside the lamp. Compared to an incandescent lamp, a fluorescent lamp is far more efficient at converting electrical energy into usable light.
To know more about the fluorescent light visit:
https://brainly.com/question/8979272
#SPJ1
If 200,4 g of water is mixed with 101.42 g of salt, the mass of the final solution
would be reported as
A) 302 8.
B) 3008
C) 301.8 8.
D) 301.828
Answers
Answer:
C) 301.8
Explanation:
Hello!
In this case, since the solution is composed by water and salt, the total resulting mass comes up from adding the mixed mass of each one:
\(m_{sol}=m_{w}+m_{salt}\)
Thus, we plug in to obtain:
\(m_{sol}=200.4g+101.42g\\\\m_{sol}=301.8g\)
Therefore, the answer should be C) 301.8 as it has four significant figures.
Best regards!
NEED ANSWER FAST 50 POINTS
A mixture of copper sulfate and water is heated, leaving a residue of copper sulfate in the container. Which method was used to separate the mixture?
A. chromatography
B. evaporation
C. filtration
D. distillation
Answers
The method used to separate copper sulfate and water mixture was evaporation.
Explanation:
Chromatography is a separation technique used in labs. In this technique, there are two phases, the mobile phase, and the stationary phase. The phase in which the mixture is dissolved is termed as mobile phase and the phase which serves as a carrier through the system like, sheet, capillary, etc. is termed as mobile phase.Evaporation is a process in which the action of heat is employed to separate dissolved solids from liquid. Due to heat liquid gets evaporated leaving the solid behind.Filtration is a process in which insoluble particles are separated from the liquid by allowing them to pass through a porous substance such as filter paper. Distillation is a process used in the separation of the mixture of liquids with different boiling points.So, from this, we can conclude that the method used to separate copper sulfate and water mixture was evaporation.
Learn more about separation techniques here
brainly.com/question/625109?referrer=searchResults
brainly.com/question/16774902?referrer=searchResults
Answer:
Just did this on my test the answer is evaportion.
Explanation:
PLZ HELP ME BEING TIMED!!!!!
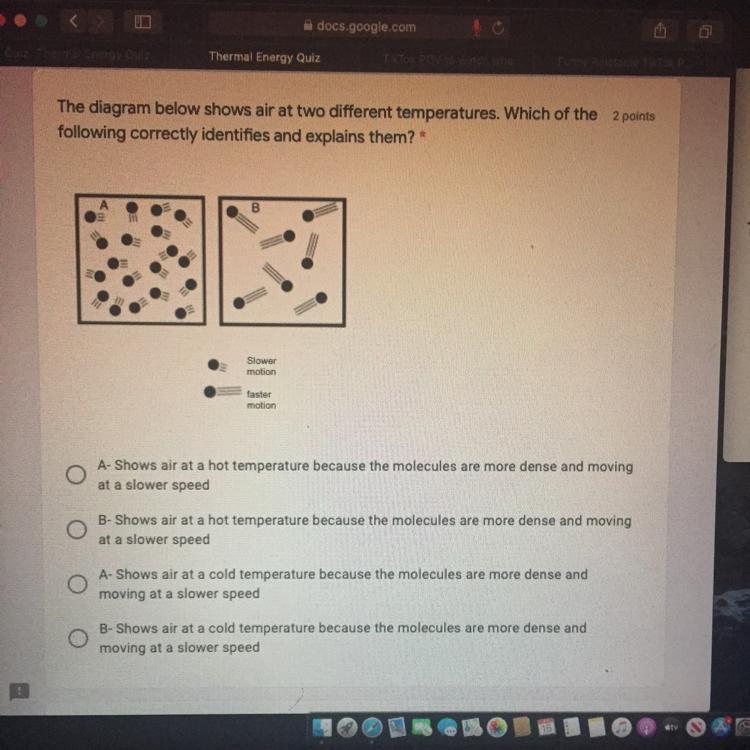
Answers
19. The interaction between matter and energy is called
A. Energy
B. Work
C. Power
D. None of the above
Answers
Answer:
Physics
Explanation:
D
How did he show that these particles had a charge on them?
Answers
J.J. Thomson discovered electrons and their negative charge through the cathode ray experiment, leading to the development of the plum pudding model of the atom.
J.J. Thomson, a British physicist, was the first to discover electrons in 1897.
He conducted the cathode ray experiment to identify the negatively charged particles.
The cathode ray tube is a vacuum-sealed glass tube with two electrodes at each end: a cathode and an anode.
When a high voltage electrical current is applied to the electrodes, the tube glows, indicating that the cathode rays are being emitted from the cathode and traveling through the tube towards the anode.
The cathode rays were found to have a negative charge, according to Thomson.
These rays were identified as particles by the presence of a magnet, which caused the particles to bend in the direction opposite to the magnet's polarity.
This discovery indicated that the particles had a charge on them because they were deflected by the magnetic field, which is only possible if the particles have an electric charge.
Thomson further concluded that these particles were about 1,000 times smaller than hydrogen atoms because of the degree of deflection they experienced in the magnetic field.
Furthermore, Thomson created the plum pudding model of an atom, in which electrons are dispersed throughout a positively charged matrix, based on his findings.
For more such questions on electrons
https://brainly.com/question/26084288
#SPJ8
Which answer choice is a way to speed up how fast a solute can dissolve in a solvent? A: heat
B: mass C: density D: cold
Answers
a molecule that functions as the electron donor in a redox reaction ______
Answers
The atom or molecule that contributes electrons—in this case, magnesium is known as the reducing agent because the reduction of another molecule is made possible by the electrons it donates.
What is the electron acceptor in a redox reaction?a molecule that, during a redox reaction, takes or absorbs electrons from another molecule. In a redox reaction, an electron acceptor reduces itself and acts as an oxidizing agent. Oxygen, nitrate, iron (III), manganese (IV), sulfate, carbon dioxide, and other elements are examples of acceptors.
The electron donor is the reducing agent, right?A chemical species that "donates" an electron to an electron recipient is referred to as a reducing agent in chemistry. This chemical species is also referred to as a reductant, reducer, or electron donor (called the oxidizing agent, oxidant, oxidizer, or electron acceptor).
To know more about redox reaction visit:-
https://brainly.com/question/13293425
#SPJ4
C6H12O6 + 602 → 6CO2 + 6H₂O
The most efficient ratio is
1 C6H12O6 6 02.
Which set of reactants will be the most
efficient (least wasteful of materials) for
the reaction?
A. 1.0 mol C6H12O6 and 3.0 mol O₂
B. 1.5 mol C6H₁2O6 and 3.0 mol O₂
C. 3.0 mol C6H₁2O6 and 6.0 mol O₂
D. 0.5 mol C6H₁2O6 and 3.0 mol O₂
Answers
Answer:
D
Explanation:
The ratio of C6H12O6 (which will be referred to as "the carb") to oxygen is 1 to 6, so if we find an answer which has the same ratio, it should be chosen. A is 1:3
B is even worse with a ratio of the carb to oxygen of 1:2
C is the same as B, 1:2
D has a ratio of the carb to oxygen of 1:6, which is what we are looking for.
Identify the substance that has formula mass of 133.5amu.
(a) MgCI
b)SCI
c)BCI
D) AICI
Answers
The calculated formula masses to 133.5 amu, we find that the substance with a formula mass closest to 133.5 amu is (d) AlCl3. Therefore, the answer is option D.
To identify the substance with a formula mass of 133.5 amu, we need to calculate the formula mass of each given substance and compare it to 133.5 amu.
(a) MgCl2:
The formula mass of MgCl2 can be calculated by adding the atomic masses of magnesium (Mg) and chlorine (Cl).
Mg: atomic mass = 24.31 amu
Cl: atomic mass = 35.45 amu
Formula mass of MgCl2 = (24.31 amu) + 2(35.45 amu) = 95.21 amu
(b) SCl:
The formula mass of SCl can be calculated by adding the atomic masses of sulfur (S) and chlorine (Cl).
S: atomic mass = 32.07 amu
Cl: atomic mass = 35.45 amu
Formula mass of SCl = 32.07 amu + 35.45 amu = 67.52 amu
(c) BCl:
The formula mass of BCl can be calculated by adding the atomic mass of boron (B) and chlorine (Cl).
B: atomic mass = 10.81 amu
Cl: atomic mass = 35.45 amu
Formula mass of BCl = 10.81 amu + 35.45 amu = 46.26 amu
(d) AlCl3:
The formula mass of AlCl3 can be calculated by adding the atomic mass of aluminum (Al) and 3 times the atomic mass of chlorine (Cl).
Al: atomic mass = 26.98 amu
Cl: atomic mass = 35.45 amu
Formula mass of AlCl3 = 26.98 amu + 3(35.45 amu) = 133.78 amu. Option D
For more such questions on masses visit:
https://brainly.com/question/24191825
#SPJ8
You are out hiking on a cold snowy day. You put on your battery-heated socks. In which direction is the thermal energy flowing?
There is no thermal energy in this scenario.
Thermal energy is moving from the air to your socks
Thermal energy is moving from your feet to your socks
Thermal energy is moving from your socks to your feet
Answers
The correct answer is that thermal energy is moving from your feet to your socks. The battery-heated socks work by using the heat generated by your body to warm your feet in cold weather.
The body produces heat, which is converted into thermal energy, and moves from your feet to the socks.
In order to warm your feet and make them more comfortable in cold weather, the socks use thermal energy. The thermal energy is only transferred in one direction, from your feet to the socks.
No more energy is produced because the battery-heated socks utilise your body's thermal energy to keep your feet warm.
To learn more about thermal energy visit:
https://brainly.com/question/19666326
#SPJ1
Consider the reaction: CO (g) + 2 H2 (g) ⇌ CH3OH (g) where the Kp is 2.26 x 10^4 at 25°C. Calculate ΔGrxn for the reaction at 25°C under standard conditions.
Answers
The standard Gibbs free energy change of the reaction at 25°C is -18,262 J/mol.
What is energy?Energy is the ability to do work. It exists in many forms, such as kinetic energy (energy of motion), potential energy (stored energy), thermal energy (heat), chemical energy (energy stored in the bonds of molecules), electrical energy (energy carried by electrons), and nuclear energy (energy produced in the nucleus of an atom).
The reaction given is a reversible reaction and the given Kp value is at 25°C. Therefore, we can use the equation ΔG°rxn = -RT ln Kp to calculate the standard Gibbs free energy change of the reaction.
We know that R = 8.314 J/mol K and T = 298 K (25°C)
Therefore, ΔG°rxn = - 8.314 J/mol K x 298 K x ln (2.26 x 10⁴)
ΔG°rxn = -18,262 J/mol
Therefore, the standard Gibbs free energy change of the reaction at 25°C is -18,262 J/mol.
To learn more about energy
https://brainly.com/question/29339318
#SPJ1
What is the atomic number?
pls help.

Answers
Answer:
8
Explanation: