What does the glycosidic linkage of 2 glucose molecules change the glucose from and into?
Answers
The glycosidic linkage of 2 glucose molecules changes the glucose from a monosaccharide into a disaccharide. A monosaccharide is a simple sugar, such as glucose, fructose, or galactose, that cannot be further hydrolyzed to yield smaller sugars.
In the case of glucose, when two glucose molecules undergo a condensation reaction, a glycosidic linkage is formed between the anomeric carbon of one glucose molecule and a hydroxyl group on the other glucose molecule. This results in the formation of a β-D-glucopyranosyl-(1→4)-β-D-glucopyranose molecule, which is also known as maltose.
Maltose is a reducing sugar, which means that it can undergo oxidation reactions and can be detected by tests such as the Benedict's test. It is commonly found in grains, such as barley and wheat, and is used in the production of beer and other fermented beverages.
To know kore about glycosidic linkage visit:-
https://brainly.com/question/28459643
#SPJ11
Related Questions
When you watch a sunset, is the Sun really moving across the sky? What's happening?
(Science)
Answers
A 6 kg rock rolls down a hill with a momentum of 12 kg m/s. Work out the velocity of the rock.
Answers
momentum = mass x velocity
When know that:
momentum is 12 km m/s
Mass is 6 kg
Hence, velocity = 12/ 6 = 2m/s
If the total amount of energy required to change a substance to a different phase and temperature is positive, then does that mean that the
phase change has moved in the direction of solid gas or gas - solid and has the temperature increased or decreased?
A. solid-gas temperature increase
B. solid-gas temperature decrease
C. gas sold temperature increase
D. gas - solid temperature decrease
Answers
how many significant figures are in 823.012
Answers
Name a liquid substance that could be used in the laboratory for dissolving dry mortar on floor
Answers
Acetone is a liquid substance that could be used in the laboratory for dissolving dry mortar on the floor.
Acetone is a commonly used solvent in laboratories due to its ability to dissolve a wide range of substances, including adhesives and resins. It is a volatile and flammable liquid with a low boiling point, making it convenient for cleaning purposes. Acetone is effective in dissolving dried mortar on floors as it can break down the cementitious components and facilitate their removal.
Acetone is a liquid substance that could be used in the laboratory for dissolving dry mortar on the floor.
You can learn more about Acetone at
https://brainly.com/question/2174621
#SPJ11
How much energy does a wave with a frequency of 7.0 x 1012 Hz have
Answers
Answer:
4.64 × 10⁻²¹ J/s
Explanation:
[Only if the wave travels at the speed of light]
Because a wave presumed to be at light speed is a photon, E = hf.
If frequency is not given, E = hc/λ
c is the speed of light in meters per second. c = 299, 792, 458 m/s.
λ is the wavelength which is dependent.
h or plancks constant = 6.62607004 × 10⁻³² m² kg / s.
E is in units of J / s or jules ( m² kg ) per second.
If the wave has a frequency of 7.0 x 10¹² Hz or 7 THz (Tera-Hertz).
E = (6.62607004 × 10⁻³⁴) × (7.0 × 10¹²) =
46.38249028 × 10⁻²² =
4.638249028 × 10⁻²¹ J/s ≈
4.64 × 10⁻²¹ J/s
Enter the balanced complete ionic equation for the acid-base reaction
Express your answer as a chemical equation including phases.
HCHO₂ (aq) + KOH(aq) →
Answers
Balanced complete ionic equation for the acid-base reaction is
HCHO₂ (aq) + KOH(aq) → KCHO₂(aq) + H₂O(l)
Ionic equation is a chemical equation in which the electrolytes in aqueous solution are expressed as dissociated ions
Here given equation is
HCHO₂ (aq) + KOH(aq) →
Then we have to complete this equation to write the product then
HCHO₂ (aq) + KOH(aq) → KCHO₂(aq) + H₂O(l)
Then break all the electrolyte into the ion
H⁺ + CHO₂⁻ + K⁺ + OH⁻→ K⁺ + CHO₂⁻ + H₂O
Then cancel the spectator ion on both side then
CHO₂⁻ and K⁺
Know more about ionic equation
https://brainly.com/question/14041637
#SPJ1
Heavier elements will undergo fission in order to:
a increase total binding energy
b decrease total binding energy
c increase binding energy per nucleon
d decrease binding energy per nucleon
Answers
Heavier elements will undergo fission in order to b. decrease total binding energy. Fission is a nuclear reaction in which a heavy nucleus splits into two or more lighter nuclei.
During this process, some of the mass is converted into energy, which can be harnessed to generate electricity. However, fission also results in a decrease in the total binding energy of the nucleus.
The total binding energy of a nucleus is the energy required to break apart all of its nucleons (protons and neutrons). It is a measure of the stability of the nucleus. Heavier elements have higher total binding energies, but they are also less stable. By undergoing fission, these elements can reduce their total binding energy and become more stable.
Fission also results in an increase in binding energy per nucleon. This is because the lighter nuclei produced by fission have a higher binding energy per nucleon than the original nucleus. This increase in binding energy per nucleon is what releases energy during the fission process.
In summary, heavier elements undergo fission in order to decrease their total binding energy and become more stable. This process also results in an increase in binding energy per nucleon, which releases energy that can be used for practical applications.
To learn more about nuclear reaction, refer:-
https://brainly.com/question/13315150
#SPJ11
Answer: increase binding energy per nucleon
What are the three mistakes in the set up below

Answers
In the diagram, we see a distillation process. This is a separation process that takes advantage of differences in the boiling point of a mixture of liquids, so the more volatile liquid will evaporate and be recovered as distillate.
1) The first error seen is that the liquid solution mixture is not heated. In order to separate the liquids, we must heat the mixture by adding heat, and this heat is represented in the diagram with a flame.
2) The second mistake is the position of the thermometer, the thermometer must not be in contact with the liquid, it must be at the height of the bulb so that it is in contact with the vapor.
3) The third error is the exit of the coolant water. The water exchanges heat with the steam, so the steam cools and condenses and the water absorbs the heat, and its temperature increases, so it does not come out cold.
In the following figure we can see the mistakes:

Help me with this page please due today!

Answers
The ZnCl2 has a mass of 52.4 g.
The mass of NH3 is 12.24 g
How do we obtain it?Knowing that,
Zn number of moles is 25 g/65 g/mol.
= 0.385 mole
If 1 mole of zinc creates 1 mole of zinc chloride
0.385 mole of ZnCl2 is thus created.
ZnCl2 mass produced is 0.385 mole * 136 g/mol.
= 52.4 g
2) The formula for N2's moles is 10g/28 g/mol, or 0.36 moles.
30 moles of H2 are equal to 60 g divided by 2 g/mol.
If 3 moles of H2 and 1 mole of N2 react,
N2 interacts with 0.36 moles at 0.36 * 3/1.
= 1.08 moles
N2 is hence the limiting reactant.
2 moles of NH3 are produced from 1 mole of N2.
The result of 0.36 moles of N2 is 0.36 * 2/1.
= 0.72 moles
NH3 mass equals 0.72 moles times 17 g/mol.
= 12.24 g
Learn more about stoichiometry:brainly.com/question/30215297
#SPJ1
At which position in its orbit was the Moon located during the 2015 supermoon total lunar eclipse?

Answers
The moon was located at position C in its orbit during the 2015 supermoon total lunar eclipse.
What is a supermoon lunar eclipse?A supermoon can be described as a full moon that nearly coincides with perigee which is the closest that the Moon comes to the Earth in its elliptic orbit as results in a slightly larger-than-usual apparent size of the lunar disk can be viewed from the earth.
Perigee syzygy is the technical name of the Earth-Moon-Sun system or a full Moon around perigee. The association of the Moon with oceanic and crustal tides claims that the supermoon phenomenon can be associated with an increased risk of events such as volcanic eruptions and earthquakes, but no such connection has been found.
In the 21st century, there are 87 lunar eclipses in total, of which 6 are micro moons and 28 are supermoons. A supermoon lunar eclipse was seen in the September 2015 lunar eclipse.
Learn more about lunar eclipse, here:
https://brainly.com/question/12075389
#SPJ2
(b) The chemical equation for the reaction between lithium and oxygen is 4Li + 0,2L1,0 Write a chemical equation for the reaction between lithium and nitrogen
Answers
Answer:
Li3N
Explanation:
Li+N2=Li3N.........................
Answer:
4 Li(s)+O2(g) → 2 Li2O(s)
Explanation:
its right
Which of the following physical changes is not an example of a change in state?
A. rock breaking with a hammer C. corn with ice
B. a (ball looking) object into water D. water filling into a ice cube mold
Answers
Answer:
Option
Explanation:
Rock is broken into piece but thestate is normal
The soil organic matter in Kenya has a stable carbon isotopic composition 813C of -18
permil. Assuming that the air SIC value is -7 permil, what is the relative contribution of C3 and
C4 plants to this organic matter?
Answers
The estimated relative contribution of C3 plants is approximately 88%, while the estimated relative contribution of C4 plants is approximately 12% to the organic matter in Kenya's soil.
To determine the relative contribution of C3 and C4 plants to the organic matter in Kenya's soil, we can use the difference in stable carbon isotopic compositions (δ13C values) between these plant types.
C3 and C4 plants have distinct δ13C values due to differences in their carbon fixation pathways. C3 plants generally have δ13C values ranging from -22 to -33 permil, while C4 plants typically exhibit δ13C values from -9 to -16 permil.
Given that the stable carbon isotopic composition (δ13C) of the soil organic matter in Kenya is -18 permil, we can compare this value to the δ13C values of C3 and C4 plants to estimate their relative contributions.
Let's denote the relative contribution of C3 plants as "x" and the relative contribution of C4 plants as "y." Since the contributions of C3 and C4 plants sum up to 100%, we have the equation:
x + y = 100% (equation 1)
Now, let's assign the δ13C values to the contributions of C3 and C4 plants. Assuming the air δ13C value is -7 permil, we can write the following equations:
-18 = x * (-33) + y * (-16) + (-7) * (1 - x - y) (equation 2)
Solving equations 1 and 2 simultaneously will provide us with the relative contributions of C3 and C4 plants.
Using the given δ13C values and solving the equations, we find:
x ≈ 0.88 (or 88%)
y ≈ 0.12 (or 12%)
Therefore, the estimated relative contribution of C3 plants is approximately 88%, while the estimated relative contribution of C4 plants is approximately 12% to the organic matter in Kenya's soil.
Learn more about organic matter from the link given below.
https://brainly.com/question/31228791
#SPJ4
Do Positive ions Tend to be metals or no metals
Answers
How many moles of calcium carbonate are in 63.8 g of calcium carbonate?
Answers
Answer:
0.638 moles in 63.8g of calcium carbonate
Explanation:
An electrochemical cell used for the "Quant" purpose (that is, to find unknown concentration of the analyte) is based on: A. a battery B. an electrolytic cell C. neither A nor B D. either A or B E. can not be decided
Answers
The answer to your question is D, either A or B. An electrochemical cell can be used for quantitative analysis, also known as "quant" analysis, to determine the concentration of an unknown analyte.
Both batteries and electrolytic cells can be used for this purpose, depending on the specific setup of the electrochemical cell. Therefore, the answer is that it could be either A or B.
An electrochemical cell used for the "Quant" purpose (that is, to find unknown concentration of the analyte) is based on: C. neither A nor B. It is actually based on a galvanic cell or a potentiometric cell, which measure the potential difference between two half-cells in order to determine the concentration of the analyte.
To know mor about electrolytic cell visit:
https://brainly.com/question/4030224
#SPJ11
PLEASEEEE HELP DUE IN 2 HOURSS PLEASE!! 15 POINTS!!!!Someone decides to swap out nitric acid (HNO3) for hydrogen
chloride (HCI), given that it will be much stronger due to opposing dipole
forces. Explain if they are correct or incorrect and why.
*
Answers
Explanation:
The claim that hydrogen chloride (HCl) would be much stronger than nitric acid (HNO3) due to opposing dipole forces is incorrect.
Both HCl and HNO3 are strong acids, meaning that they dissociate completely in water to produce H+ ions. The strength of an acid is determined by the degree to which it dissociates in water. In other words, the stronger the acid, the more H+ ions it produces in water.
The dissociation of HCl and HNO3 in water can be represented as follows:
HCl + H2O → H+ + Cl-
HNO3 + H2O → H+ + NO3-
As we can see, both HCl and HNO3 produce H+ ions in water. Therefore, the strength of an acid cannot be solely determined by its dipole forces.
In addition, it's important to note that HCl is a much more volatile and corrosive acid than HNO3. It can cause severe respiratory and skin irritation when it is inhaled or comes into contact with skin. Therefore, switching HNO3 for HCl could be dangerous and should not be done without proper precautions and expert knowledge
a student collected a sample of hydrogen gas by the displacement of water. Calculate the number of molecules of water vapor in the sample of gas
Answers
Using a method known as water displacement, gases created during laboratory experiments are frequently collected. To obtain the accurate value of the gas's pressure .
The vapor pressure caused by water in a sample can be compensated for. The gas is not pure since it is combined with water vapor from evaporating water because it is gathered above water. By deducting the contribution of the water vapor, one may apply Dalton's law to determine how much of the desired gas is present: P total equals Pg plus PH 2O. The required gas's pressure is P g. Pg = P Total + P H 2 O List the known quantities and make a plan for the issue. pressure in the atmosphere. A water pan is filled with a bottle that has been turned upside down and filled with water. Rubber tubing that is attached to the reaction flask and supplied underneath the water bottle.
To learn more about pressure please click on below link
https://brainly.com/question/12971272
#SPJ4
The desert sand dunes seen here are are created through the processes of?
Answers
Balance the following chemical equations.
KNO3 + H2CO3-> K2CO3 + HNO3
Answers
Answer: 2KNO3 + H2CO3 >>> K2CO3 + 2HNO3
Explanation:
what is the set point on a thermostat? How is this idea important to feedback cycles?
Answers
Answer:
The set point on a thermostat is the desired or target temperature that a heating or cooling system is set to maintain. The thermostat continuously measures the current temperature of the room and compares it to the set point. If the current temperature is lower than the set point, the thermostat will signal the heating system to turn on, and if the current temperature is higher than the set point, the thermostat will signal the cooling system to turn on. The set point can be adjusted manually or automatically.
The idea of a set point is important to feedback cycles because it represents a reference value or goal that a system tries to achieve and maintain. In a feedback cycle, a set point provides a stable reference value that helps to regulate the system's output. The feedback loop continuously measures the output of the system and compares it to the set point. If the output is lower than the set point, the system adjusts itself to increase the output, and if the output is higher than the set point, the system adjusts itself to decrease the output.
The set point is critical for maintaining a stable feedback loop and preventing instability or oscillations in the system's output. If the set point is too high or too low, the system may oscillate around the set point or fail to achieve it altogether. Therefore, choosing an appropriate set point is essential for maintaining a stable and efficient feedback cycle in various systems, including thermostats, control systems, and biological systems.
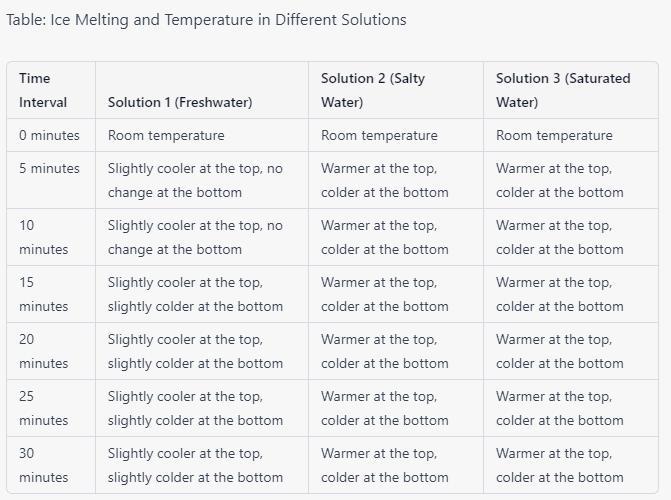
2. Complete the data table. Sketch, use your computer to draw, or write a detailed description to describe how the ice melts into the different solutions. At each interval, record the temperature at the top and bottom of the glass.

Answers
Using an Hypothetical scenario, the data table that shows the detailed description on how the ice melts into the different solutions is given in the image attached.
What is the solution about?In Solution 1 (freshwater), the ice melted slowly and evenly, resulting in a layer of cold water at the bottom of the glass. At each interval, the temperature at the top of the glass was slightly cooler than room temperature, while the temperature at the bottom remained the same.
In Solution 2 (salty water), the ice melted faster at the top than the bottom due to the denser salt water. This resulted in a layer of cold freshwater on top of the denser salty water. At each interval, the temperature at the top of the glass was warmer than the bottom.
Lastly, In Solution 3 (saturated water), the ice melted rapidly and unevenly, forming a layer of very cold freshwater on top of the denser saturated solution. At each interval, the temperature at the top of the glass was warmer than the bottom.
Learn more about freshwater from
https://brainly.com/question/898979
#SPJ1
Isomers are molecules with the same molecular formula, but different structural formulae.
How many isomers are there in C7H16?
A.6
B.7
C.8
D.9
Answers
Answer:
D 9 is the answer
Explanation:
Hope it helps you
Please mark as brainliest answerwhat minimum pressure in psi would a 250-ml aerosol can have to withstand if it were to contain 2.50 l of gas measured at 700 torr? assume the temperature remains constant.
Answers
The minimum pressure of ideal gas is 135.36 psi.
We need to know about the ideal gas theory to solve this problem. The ideal gas is assumed that there is no interaction between particles in a gas. It can be determined by the equation
P . V = n . R . T
where P is pressure, V is volume, n is the number of moles gas, R is the ideal gas constant (8.31 J/mol.K) and T is temperature.
From the question above, we know that
V₁ = 250 mL = 0.25 L
V₂ = 2.5 L
P₂ = 700 torr
When the initial and final temperature is the same, we can use the ratio of pressure and volume as
P₁ . V₁ = P₂ . V₂
P₁ . 0.25 = 700 . 2.5
P₁ = 7000 torr
convert to psi
P₁ = 7000 torr
P₁ = 7000 / 51.715 psi
P₁ = 135.36 psi
Find more on ideal gas at: https://brainly.com/question/25290815
#SPJ4
Suppose you wanted to make a buffer of exactly pH 7.00 using KH2PO4 and Na2HPO4. If the final solution was 0.1 M in KH2PO4, what concen- tration of Na2HPO4 would you need
Answers
To create a pH 7.00 buffer using KH2PO4 and Na2HPO4, a concentration of 0.1 M for Na2HPO4 would be needed.
To create a buffer with a pH of exactly 7.00 using KH2PO4 and Na2HPO4, we need to consider the pKa values of the two components. The pKa of KH2PO4 is 7.21, and the pKa of Na2HPO4 is 6.86.
Since we want a buffer with a pH of 7.00, we need the pKa values to be as close as possible. To achieve this, we should choose the ratio of [KH2PO4] to [Na2HPO4] to be 1:1.
This means that for a 0.1 M solution of KH2PO4, we would need an equal concentration of Na2HPO4, resulting in a concentration of 0.1 M for Na2HPO4 in the buffer solution. By maintaining this ratio, the buffer will resist significant changes in pH when small amounts of acid or base are added.
To know more about Na2HPO4 refer here
brainly.com/question/31393050#
#SPJ11
When sugar is fermented, alcohol is produced. What other substance is produced? Also.. What effects does this substance have in (i) baking (ii) beer brewing.
Answers
ANSWER: It's (ii) I do believe
The sugar is fermented and the ethanol and carbon dioxide are produced. The fermentation of ethanol is used in bread baking and the fermentation found in grapes makes beer.
What is fermentation?Fermentation is the mechanism by which sugar is converted into alcohol. Ethanol Fermentation can be described as a biological process that converts sugars such as glucose, sucrose, and fructose into cellular energy by producing ethanol and carbon dioxide as a side effect.
Alcoholic fermentation is called anaerobic since yeast conducts this transformation in the absence of oxygen. The fermentation of ethanol has applications such as the manufacture of alcoholic drinks, ethanol fuel manufacture, and bread baking.
Fermentation of the natural sugars can be seen in grapes creating wine or beer as well as in apples and pears producing cider and Perry, respectively. Alcoholic fermentation takes place in species of fish, where it offers energy when oxygen is scarce.
Learn more about fermentation, here:
https://brainly.com/question/13050729
#SPJ6
What is GMOs? ( Thanks btw )
Answers
Answer:
living organisms whose genetic material has been artificially manipulated in a laboratory through genetic engineering
Explanation:
How does the existence of atoms explain the conservation of matter?
Answers
The existence of atoms helps to explain the conservation of matter because atoms are the smallest unit of an element that retains the properties of that element. Atoms cannot be created or destroyed, only rearranged, which means that the total number of atoms in a closed system remains constant.
When a chemical reaction takes place, the atoms in the reactants are rearranged to form the products, but the total number of atoms in the system remains the same. This is the law of conservation of matter, which states that the total mass of the reactants in a chemical reaction must equal the total mass of the products.
The existence of atoms as the basic building blocks of matter helps to support the law of conservation of matter by providing a basis for understanding that the total number of atoms in a system remains constant, even though they may be rearranged during a chemical reaction.
Learn more about law of conservation of matter here: https://brainly.com/question/29530861
#SPJ4
plsss help, chart how ions are formed

Answers
Answer:
Magnesium-
atomic symbol: Mg
the total number of electrons: 12
group number: 2
the number of valence electrons: 2
Nitrogen:
atomic symbol: N
the total number of electrons: 7
group number: 15
the number of valence electrons: 5
Oxygen:
atomic symbol: O
the total number of electrons: 8
group number: 16
the number of valence electrons: 6
Potassium:
atomic symbol: K
the total number of electrons: 19
group number: 1
the number of valence electrons: 1
Sodium:
atomic symbol: Na
the total number of electrons: 11
group number: 1
the number of valence electrons: 1
Explanation:
Sorry that I couldn't answer the other questions, they are hard to see. I got these from the periodic table. Hope this helps!