what does not occur during the processing of eukaryotic mrna?
a.splicing the exons together
b.translation
c.addition of a cap at the 5' end
d.removal of introns
e.addition of a poly A tail at the 3' end
Answers
During the processing of eukaryotic mRNA, translation does not occur. Eukaryotic mRNA processing involves splicing the exons together, the addition of a cap at the 5' end, removal of introns, and the addition of a poly A tail at the 3' end. Therefore, the correct option is (B).
In the process of translation, the information that is coded in the mRNA is translated to a sequence of amino acids. This process occurs during protein synthesis on ribosomes in the cytoplasm. The coding sequences of eukaryotic mRNA are separated by non-coding sequences or introns, which must be removed by splicing to produce mature mRNA.
The mRNA processing events that occur in the eukaryotic nucleus include the following:
RNA capping: This is the addition of a 7-methylguanosine cap to the 5' end of the mRNA. This cap plays a role in mRNA stability, splicing, and translation by binding to the eukaryotic initiation factor 4E (eIF4E), which aids in the recruitment of the 40S ribosomal subunit and other translation initiation factors.Polyadenylation: The 3' end of the pre-mRNA is cleaved and a sequence of adenine nucleotides is added. This modification helps to stabilize the mRNA and serves as a binding site for proteins that help to export the mRNA from the nucleus.Splicing: Non-coding introns are removed from the pre-mRNA and the coding exons are spliced together to produce mature mRNA.To know more about "Eukaryotic mRNA" refer here:
https://brainly.com/question/29032099#
#SPJ11
Related Questions
Draw the structure of
CH3CH2CH2CH2CH2CHCH2
Answers
Explanation:
the last CH2 should CH3
because the first and last carbons are bonded to three hydrogens

Can someone help me please!

Answers
When any reversible reaction is at equilibrium, what conditions are necessarily true? Select one or more: O The amount of products equals the amount of reactants. O The amounts of reactants and products has stopped changing. O Reactants and products are both present in the reaction mixture. O The rate of the forward reaction equals the rate of the reverse reaction. O The conversion between reactants and products has stopped.
Answers
At equilibrium, a reversible reaction has reached a state where the rate of the forward reaction is equal to the rate of the reverse reaction. This means that the reaction has reached a point where the amounts of reactants and products have stopped changing.
Therefore, the second condition, "The amounts of reactants and products has stopped changing" is necessarily true for any reversible reaction at equilibrium.
The first condition, "The amount of products equals the amount of reactants", may or may not be true depending on the stoichiometry of the reaction and the initial amounts of reactants and products. If the reaction has a 1:1 stoichiometry, then the amount of products would be equal to the amount of reactants at equilibrium. However, if the reaction has a different stoichiometry, then the amounts of reactants and products at equilibrium would be different.
The third condition, "Reactants and products are both present in the reaction mixture", is not necessarily true as some reactions may have only one reactant or one product. For example, the reaction 2H2O(l) ↔ 2H2(g) + O2(g) has only one reactant (water) and two products (hydrogen and oxygen gases).
The fifth condition, "The conversion between reactants and products has stopped", is not a necessary condition for equilibrium. At equilibrium, the conversion between reactants and products may still be occurring, but at equal rates. This means that the concentrations of reactants and products remain constant over time.
In summary, the necessary conditions for a reversible reaction at equilibrium are that the amounts of reactants and products have stopped changing and that the rate of the forward reaction equals the rate of the reverse reaction.
Learn more about reversible reaction here:
https://brainly.com/question/16614705
#SPJ11
Which of the following is the best way to model the process of weathering?
Using a fan on a pile of sand causing the sand to move into a pile in a new location.
Using a ramp to dump sand at the bottom causing the sand to pile up.
Using a watering can sprinkle water onto sand causing the sand to move to a new location.
Filling a glass bottle with water and allowing it to freeze causing the bottle to break.
Answers
The best model of weathering is using a fan on a pile of sand causing the sand to move into a pile in a new location.
Weathering refers to the gradual breakdown of a rock due to the agents of denudation. The agents of denudation could be physical, chemical or biological.
The breakdown of rocks leads to the formation of soil. The closest model to the weathering of rock is using a fan on a pile of sand causing the sand to move into a pile in a new location.
The pile represents the rock which is being broken down as the fan blows the pile and it settles on a new location.
Learn more: https://brainly.com/question/14426457
someone pls explain how I do this work
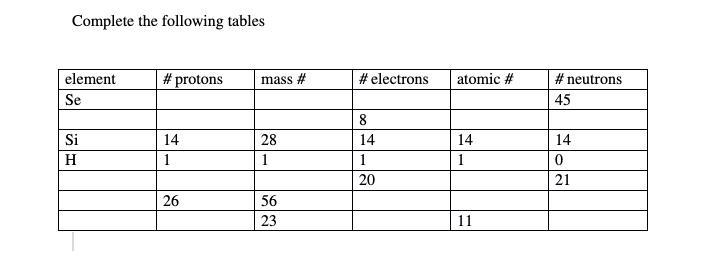
Answers
Answer:
Explanation:
Element #of protons Mass #. # of electrons Atomic #. Neutron
Se. 34. 78.96. 34 34 45
Si. 14. 28. 14. 14 14
H. 1. 1. 1 1 0
Ca. 20. 40.078. 20. 20. 21
Fe. 26. 56. 26. 26. 30
Na. 11. 23. 11. 11. 12
in a metallic bond, the electrons are free to move easily from one atom to the next throughout the metal and are not attached to a particular atom, and are said to be
Answers
Electrons are said to be delocalized electrons
Delocalized electrons are electrons in a molecule, ion, or solid metal that are not associated with a single atom or covalent connection. Electrons that have been delocalized are trapped within an orbital that spans many neighboring atoms.
Because electrons can freely roam within these molecular orbitals, each electron becomes separated from its parent atom. Delocalization refers to electrons. The strong forces of attraction between the positive nuclei and the delocalized electrons hold the metal together.
As a result, in a metallic link, electrons are free to flow freely from one atom to the next across the metal and are not bound to a specific atom; these electrons are referred to as delocalized electrons.
To learn more about delocalized electrons visit
https://brainly.com/question/18114979
#SPJ4
What is 0.866 km to mm in scientific notation
Answers
Answer:
866000
Explanation:
because one kilometer (km) = 1000000 millimeters (mm)
What is the equilibrium equation for the reaction: nh4no3(s) ⇌ n2o(g) + 2 h2o(g)?
Answers
The equilibrium equation for the reaction:
NH₄NO₃(s) ⇌ N₂O(g) + 2H₂O(g) is Kp = [ N₂O][H₂O]².
In the equilibrium expressions we only considered the gases and the aqueous compounds. When the one or the more of the substances in the system that will exists in the gaseous phase, and the partial pressure of the species which can be used for the equilibrium expression.
The chemical reaction is as :
NH₄NO₃(s) ⇌ N₂O(g) + 2H₂O(g)
The equilibrium expression is as :
Kp = [ N₂O][H₂O]².
The ratio of the concentrations for the reactants and the products is called as the equilibrium constant expression.
To learn more about equilibrium here
https://brainly.com/question/30907204
#SPJ4
Give the number of protons and the number of neutrons in the nucleus of the following isotopes: a) Carbon-14 b) Cobalt-60 c) Gold-197 d) Uranium-235
Answers
Explanation:
We are given different isotopes and we have to identify the number of protons and neutrons that are in the nuclueus of each atom.
a) Carbon-14:
By definition two isotopes are atoms that have the same atomic number but different mass number. The atomic number of an atom is equal to the number of protons of that atom, and the mass number is equal to the number of protons plus the number of neutrons.
atomic number = n° of protons
mass number = n° of protons + n° of neutrons
n° of protons = atomic number
n° of neutrons = mass number - n° of protons
n° of neutrons = mass number - atomic number
If two isotopes have the same atomic number but different mass number we can say that two isotopes have the same number of protons but different number of neutrons.
In we pay attention to carbon-14 we can look for its atomic number in the period table: 6. And its mass number is the one that we are given after the name of the element: 14.
n° of protons = atomic number = 6
n° of protons = 6
n° of neutrons = mass number - atomic number = 14 - 6
n° of neutrons = 8
b) Cobalt-60:
atomic number = 27 (from the periodic table)
mass number = 60
n° of protons = atomic number = 27
n° of protons = 27
n° of neutrons = mass number - atomic number = 60 - 27
n° of neutrons = 33
c) Gold-197:
atomic number = 79 (from the periodic table)
mass number = 197
n° of protons = atomic number = 79
n° of protons = 79
n° of neutrons = mass number - atomic number = 197 - 79
n° of neutrons = 118
d) Uranium-235:
atomic number = 92 (from the periodic table)
mass number = 235
n° of protons = atomic number = 92
n° of protons = 92
n° of neutrons = mass number - atomic number = 235 - 92
n° of neutrons = 143
Answer:
a) Carbon-14: n° of protons = 6 n° of neutrons = 8
b) Cobalt-60: n° of protons = 27 n° of neutrons = 33
c) Gold-197: n° of protons = 79 n° of neutrons = 118
d) Uranium-235: n° of protons = 92 n° of neutrons = 143
Consider the five-day demands of five local branches (Z, Y, X, W, and V) in an insurance company below: Day Z Y X W V 1 1 3 2 5 1 2 1 3 4 4 2 3 3 3 4 3 3 4 5 3 3 2 4 5 5 3 2 1 5 Demands of which branch show the highest variability?
Answers
To determine which branch shows the highest variability, we need to calculate the standard deviation of the demands for each branch. The formula for calculating the standard deviation is quite complex, but we can use a spreadsheet program like Microsoft Excel to make the calculation easier.
To determine which branch shows the highest variability, we need to calculate the standard deviation of the demands for each branch. The formula for calculating the standard deviation is quite complex, but we can use a spreadsheet program like Microsoft Excel to make the calculation easier.
Using Excel, we can enter the data for each branch into separate columns, and then use the STDEV.S function to calculate the standard deviation. Doing this, we get the following results:
- Branch Z: Standard deviation = 1.26
- Branch Y: Standard deviation = 0.89
- Branch X: Standard deviation = 0.63
- Branch W: Standard deviation = 0.63
- Branch V: Standard deviation = 1.26
From these results, we can see that Branch Z and Branch V have the highest variability in demands, with a standard deviation of 1.26. This suggests that these branches experience a wider range of demand than the other branches, which could have implications for how the company manages its resources and staff.
In conclusion, using the standard deviation to measure variability, we can see that Branches Z and V show the highest variability in demand for this insurance company.
To know more about standard deviation visit: https://brainly.com/question/13498201
#SPJ11
Using the standard deviation to measure variability, we can see that Branches Z and V show the highest variability in demand for this insurance company.
How to determine which branch shows the highest variability?To determine which branch shows the highest variability, we need to calculate the standard deviation of the demands for each branch. The formula for calculating the standard deviation is quite complex, but we can use a spreadsheet program like Microsoft Excel to make the calculation easier.
Using Excel, we can enter the data for each branch into separate columns, and then use the STDEV.S function to calculate the standard deviation. Doing this, we get the following results:
- Branch Z: Standard deviation = 1.26
- Branch Y: Standard deviation = 0.89
- Branch X: Standard deviation = 0.63
- Branch W: Standard deviation = 0.63
- Branch V: Standard deviation = 1.26
From these results, we can see that Branch Z and Branch V have the highest variability in demands, with a standard deviation of 1.26. This suggests that these branches experience a wider range of demand than the other branches, which could have implications for how the company manages its resources and staff.
In conclusion, using the standard deviation to measure variability, we can see that Branches Z and V show the highest variability in demand for this insurance company.
To know more about standard deviation visit:
brainly.com/question/13498201
#SPJ4
Lattice energy is an estimate of the bond
conductivity
group
length
strength
Answers
Answer:
Lattice energy is an estimate of the bond strength.
Determine the partial pressure and number of moles of each gas in a 16.75L vessel at 30 degree C containing a mixture of xenon and neon gases only. The total pressure in the vessel is 7.10 atm, and the mole fraction of xenon is 0.721.
What is the partial pressure of xenon?
What is the partial pressure of neon?
What is the number of moles of xenon?
What is the number of moles of neon?
Answers
First, we will calculate the number of moles of mixture of Xenon and Neon gases.Number of moles of mixture of Xenon and Neon gases:
Let x be the mole fraction of Neon.
Therefore, (1 - x) is the mole fraction of Xenon
.Mole fraction of Neon + Mole fraction of Xenon = 1x + (1 - x) = 1x = 1 - (1 -
x = 0 + x
x = 0.279
Mole fraction of Neon = 0.279
Mole fraction of Xenon = 0.721
Number of moles of gas = (Total Pressure * Volume)/(Gas Constant * Temperature)
Number of moles of Xenon = (7.10 atm * 16.75L * 0.721)/(0.08206 * (273 + 30))
Number of moles of Xenon = 8.44 moles
Number of moles of Neon = (7.10 atm * 16.75L * 0.279)/(0.08206 * (273 + 30))
Number of moles of Neon = 3.29 moles
Now, we can calculate the partial pressure of Xenon and Neon.
Partial pressure of Xenon:
Partial Pressure of Xenon = Mole fraction of Xenon * Total Pressure
Partial Pressure of Xenon = 0.721 * 7.10 atm
Partial Pressure of Xenon = 5.12 atm
Partial pressure of Neon
Partial Pressure of Neon = Mole fraction of Neon * Total Pressure
Partial Pressure of Neon = 0.279 * 7.10 atm
Partial Pressure of Neon = 1.98 atm
Learn more about atoms at
https://brainly.com/question/33049833
#SPJ11
3.00 g of AgNO3 is used to make how many moles of Ag with excess Cu
Cu + AgNO3 à Cu(NO3)2 + Ag
Answers
According stoichiometry and balanced chemical equation,as 339.74 g produces 2 moles , therefore 3 g is produced by 0.017 moles of silver.
What is stoichiometry?Stoichiometry is the determination of proportions of elements or compounds in a chemical reaction. The related relations are based on law of conservation of mass and law of combining weights and volumes.
Stoichiometry is used in quantitative analysis for measuring concentrations of substances present in the sample.
In the given example, according to balanced chemical equation as 339.74 g of silver nitrate produces 2 moles,
∴3 g of silver nitrate will produce 3×2/339.74=0.017 moles.
Therefore, 3.00 g of AgNO₃ is used to make 0.017 moles of silver.
Learn more about stoichiometry,here:
https://brainly.com/question/28780091
#SPJ1
Antacid has a pH of 11. What does that tell you about its concentration of hydrogen ions? What does that tell you about its concentration of hydroxide ions?
Answers
Answer: The concentration of hydrogen ions is \(10^{-11}M\) and the concentration of hydroxide ions is \(10^{-3}M\). Thus the concentration of hydroxide ions is more than the concentration of hydrogen ions.
Explanation:
pH or pOH is the measure of acidity or alkalinity of a solution.
pH is calculated by taking negative logarithm of hydrogen ion concentration.
\(pH=-\log [H^+]\)
\(pOH=-\log[OH^-]\)
Putting in the values:
\(11=-\log[H^+]\)
\([H^+]=10^{-11}M\)
\(pH+pOH=14\)
\(pOH=14-11=3\)
\(3=-log[OH^-]\)
\([OH^-]=10^{-3}M\)
Thus the concentration of hydroxide ions is more than the concentration of hydrogen ions.
How many atoms are in 9.01 grams of
Beryllium (Be)?
Answers
Answer:
the atomic number is 4
Explanation:
the atomic number is 4, thus beryllium contains 4 electrons and 4 protons.
a mixture of argon and xenon gases is maintained in a 7.66 l flask at a pressure of 2.44 atm and a temperature of 86 °c. if the gas mixture contains 17.0 grams of argon, the number of grams of xenon in the mixture is
Answers
By using the general gas equation, the mass of Xenon in the mixture is found to be 27.44g.
The ideal gas law, also known as the general gas equation, says that the product of the pressure and volume of one gram of an ideal gas is equal to the result of the universal gas constant and the absolute temperature of the gas.
PV = nRT denotes the general gas equation. In this equation, the letters P, V, n, R, T stand for the pressure of the ideal gas, the volume of the ideal gas, the total amount of the ideal gas given in moles, the universal gas constant and the temperature, respectively.
The general gas equation states that one mole of an ideal gas may occupy a volume of 22.414 liters under standard circumstances, which are a pressure of 101,325 Pa / 1atm and a temperature of 273.15 K or 0°C.
Given:
Pressure, P = 2.44atm
Temperature, T = 86+273 = 359K
Volume, V = 7.66L
To find:
Mass of Xe = ?
Formula:
PV = nRT
Calculations:
Let total no. of moles of Ar and Xe = y
n = PV/RT
n = 2.44 x 7.66 / (0.0821 x 359) = 0.634mol
No. of moles of Argon (yAr) = 17/40 = 0.425mol
y = yAr + yXe
0.634 = 0.425 + yXe
yXe = 0.634 - 0.425
yXe = 0.209mol
Mass of Xe = no. of moles x gram atomic mass
Mass of Xe = 0.209 x 131.293 = 27.44g
Result:
27.44g Xenon is present in the given Argon and Xenon mixture.
Learn more about General gas equation here:
https://brainly.com/question/13177371
#SPJ4
Light can travel through many materials. What happens when light passes from air into the water?
Answers
Answer: The light bends closer towards the normal and is refracted.
Explanation: The light is passing through something less dense (air) to more dense (water), it's going to bend the light more towards the normal and will refract the light making it look bent at the surface of the water.
which statement about non-digestible carbohydrates is false?
Answers
The false statement would be statement B) "They provide a significant amount of calories." Non-digestible carbohydrates do not provide significant calories since they are not broken down and absorbed by the body. Therefore, statement B) is false.
To identify the false statement about non-digestible carbohydrates, we need to consider their characteristics and properties. Here are some common characteristics of non-digestible carbohydrates, also known as dietary fiber:
1. They are resistant to enzymatic digestion: Non-digestible carbohydrates cannot be broken down by the enzymes present in the human digestive system.
2. They provide little to no caloric value: Since they are not digested, non-digestible carbohydrates generally do not contribute significant calories to the diet.
3. They promote bowel regularity: Non-digestible carbohydrates add bulk to the stool, aiding in the movement of food through the digestive system and preventing constipation.
4. They can be fermented by gut bacteria: Certain types of non-digestible carbohydrates, such as soluble fibers, are fermented by beneficial gut bacteria in the large intestine, leading to the production of short-chain fatty acids.
The complete question should be:
which statement about non-digestible carbohydrates is false?
A) They are resistant to enzymatic digestion.
B) They provide a significant amount of calories.
C) They promote bowel regularity.
D) They cannot be fermented by gut bacteria.
Learn more about carbohydrates at https://brainly.com/question/20290845
#SPJ11
Which tool would a scientist use to hold, mix, and heat chemicals in an experiment?
1. Forceps
2. a hot plate
3. a test tube
4. a thermometer
Answers
Answer:
If it's multiple choice it's 2 and 3, if not I think the best answer is 2, but I'm not exactly sure.
Draw a Lewis structure showing how two oxygen atoms share two pairs of electrons.
Answers
Answer:
See explanation
Explanation:
A Lewis or dot structure shows the electrons on the valence shells of the bonding atoms as dots.
The method was developed by Sir G.N Lewis.
Oxygen is a diatomic molecule as shown in the image attached to this answer. There are two unshared electrons on each oxygen atom as well as four shared electrons

The sharing of electrons between the two oxygen atoms results in the completion of the octet and thereby stabilizing the atoms.
Oxygen has been a group 6A element consisting of 6 valence electrons. In order to attain the stable configuration, the atom of oxygen has been required to gain two electrons.
The oxygen shares its electrons with another oxygen atom and forms a covalent bond. The resulted bond has been able to stabilize the structure.
The image attached shows the lewis dot structure of the oxygen atom. The sharing of electrons between the two oxygen atoms results in the completion of the octet and thereby stabilizing the atoms.
For more information about the lewis dot structure, refer to the link:
https://brainly.com/question/4144781

To determine the melting point of ice, which procedure would best help measure the melting point temperature?
A - Mix ice with water until just before equilibrium is reached but some ice remains that is still melting.
B - Mix ice with water until equilibrium is reached but some ice remains that is still melting.
C - Mix ice with water until equilibrium is reached but some ice remains that is no longer melting.
D - Mix ice with water until just before equilibrium is reached but some ice remains that is no longer melting.
Answers
Answer:
Explanation:
C - Mix ice with water until equilibrium is reached but some ice remains that is no longer melting.
Have Great day :)
How much heat is needed to warm 250g of water from 22℃ to 98℃?

Answers
Answer:
Thus, the required heat is 79kJ 79 k J .
Explanation:
i think its the right bottem one
GIVING BRAINLIEST!
Please define the following in your own words:
law of conservation of matter
Answers
Answer:
Matter says that the amount of matter stays the same, even when matter changes form. Sometimes it may seem that matter disappears during a science experiment, but this law tells us that matter cannot magically appear or disappear, it simply changes from one form to another.
Explanation:
brainliest?
Answer: Hope this helps:)
The Law of Conservation of Mass dates from Antoine Lavoisier's 1789 discovery that mass is neither created nor destroyed in chemical reactions. In other words, the mass of any one element at the beginning of a reaction will equal the mass of that element at the end of the reaction. The law of conservation of matter states that in any given system that is closed to the transfer of matter, the amount of matter in the system stays constant. The law of conservation of matter says that in chemical reactions, the total mass of the products must equal the total mass of the reactants. French chemist A. Lavoisier laid the foundation to the scientific investigation of matter by describing that substances react by following certain laws. These laws are called the laws of chemical combination. Matter says that the amount of matter stays the same, even when matter changes form. Sometimes it may seem that matter disappears during a science experiment, but this law tells us that matter cannot magically appear or disappear, it simply changes from one form to another. Matter can change form through physical and chemical changes, but through any of these changes, the matter is conserved. The same amount of matter exists before and after the change—none is created or destroyed. This concept is called the Law of Conservation of Mass.
How do you determine if a polyatomic ion needs to be counted together?
Answers
Answer:
Calculate from Oxidation Number
The oxidation number of oxygen is -2, and the oxidation number of hydrogen is +1. Add together the oxidation numbers of all the atoms in the polyatomic ion. In the example, -2 +1 = -1. This is the charge on the polyatomic ion.
ALEX RODE HIS BIKE
60 km in 4 Hours. How FAST WAS HE GOING
Answers
Answer:
15 km/hour
Explanation:
You need to find how fast he was going per hour.
Answer: 15
Divide. 60 ÷ 4
This gives us 15. So, Alex was going 15 mph
Select the correct answer
Giving brainliest

Answers
Answer:
pretty sure -10 degrees
Explanation:
no explanation
why the mixture of bromine and ethane is discoloured when left in the sun
Answers
Answer:
In the presence of UV light, ethane will react with bromine in a substitution reaction. UV light is the condition under which the reaction will occur so it is written above the arrow in the chemical equation. As the reaction proceeds, the intensity of the re-brown colour of the bromine water decreases.
Answer:
here ya go
Explanation:
combining bromine and ethane leads to a brown gas or liquid which is made of the elements. it is a gas at room temperature and does not affect human beings. there are many uses for bromine compounds but they are mainly used for the manufacturing of chlorine, fire retadants, water purification, graphy chemicals and pharmaceutcals. it is a naturally occurring element that can be found in animals such as sharks since they produce their own.
Calculate the number of moles in 3.01 x 10²² atoms of calcium.
Answers
Answer:
Using dimensional analysis:
3.01x1022 molecules CO2 x 1 mol CO2/6.02x1023 molecules x 44. g CO2/mole = 2.20 g CO2
Explanation:
True or False.
Polymerization can be addition or condensation.
Answers
Choose the answer that best describes HCO3^-. a proton donor a bicarbonate ion a weak acid common in the liver
Answers
HCO3^- is best described as ao bicarbonate in.
The bicarbonate ion, HCO3^-, consists of one hydrogen atom (H+), one carbon atom (C), and three oxygen atoms (O) bonded together. It is a polyatomic ion that plays a crucial role in various biological and chemical processes. Bicarbonate ions are commonly found in the body and are involved in maintaining acid-base balance, particularly in blood and cellular environments.
In terms of acidity, HCO3^- can act as a weak acid. It has the ability to donate a proton (H+) in certain chemical reactions, contributing to the regulation of pH levels in the body. However, it is important to note that HCO3^- is primarily known as a bicarbonate ion and is more commonly involved in its role as a base rather than an acid.
In summary, HCO3^- is best described as a bicarbonate ion, which is involved in maintaining acid-base balance and acts as a weak acid in specific reactions describes HCO3^-. a proton donor a bicarbonate ion a weak acid common in the liver
Learn more about bicarbonate from the given link https://brainly.com/question/32692550
#SPJ11.
HCO3^- is known as the bicarbonate ion. It acts as a weak acid or a proton donor, assisting with pH regulation in the blood by buffering acid wastes from metabolic processes. It is also involved in respiratory regulation of acid-base balance.
Explanation:HCO3^- is known as bicarbonate ion. It can act as a proton donor, thus making it a weak acid. In the body, bicarbonate ions and carbonic acid exist in a 20:1 ratio, helping to maintain blood pH balance. Bicarbonate ions prevent significant changes in blood pH by capturing free ions. During metabolic processes that release acid wastes such as lactic acid, bicarbonate ions help to buffer the acidity. These ions are even involved in respiratory regulation of acid-base balance, as they are crucial to the balance of acids and bases in the body by regulating the blood levels of carbonic acid. The stronger the acidic substance, the more readily it donates protons (H*). In contrast, bicarbonate is a weak base, meaning that it releases only some hydroxyl ions or absorbs only a few protons. Overall, the bicarbonate ion plays a critical role in various biological reactions and maintaining homeostasis.
Learn more about Bicarbonate Ion here:https://brainly.com/question/33441745
#SPJ11