Answers
Taking into account the rule of three, you can drive your new Prius hybrid a distance of 868 miles.
Rule of threeRule of three is a way of solving problems of proportionality between three known values and an unknown value, establishing a relationship of proportionality between all of them.
If the relationship between the magnitudes is direct, the direct rule of three must be applied as follow, being a, b and c known data and x the variable to be calculated:
a ⇒ b
c ⇒ x
So: x= (c×b) ÷a
Distance in miles in this caseIn this case, you can apply the following rule of three: If the car for 1 gallon of gasoline gets 56 miles, for 15.5 gallons of gasoline it gets how many miles?
1 gallon ⇒ 56 miles
15.5 gallons ⇒ x
So: x= (15.5 gallons ×56 miles) ÷1 gallon
Solving:
x= 868 miles
In summary, you can drive your new Prius hybrid a distance of 868 miles.
Learn more with this example:
brainly.com/question/12482948
#SPJ1
Related Questions
Calculate the enthalpy change for the reaction C2H4(g) + H2(g) -> C2H6(g) from the following data: Show your work.

Answers
The enthalpy change for the reaction \(C2H4(g) + H2(g) → C2H6(g)\) is -137.15 kJ/mol.
Given:C2H4(g) + H2(g) → C2H6(g)The enthalpy of formation of C2H6(g) is -84.68 kJ mol-1The enthalpy of formation of C2H4(g) is 52.47 kJ mol-1The enthalpy of formation of H2(g) is 0 kJ mol-1Hence, using Hess's Law, the enthalpy change for the reaction \(C2H4(g) + H2(g) → C2H6(g)\) can be calculated by considering the formation of reactants and products from their respective elements. It can be given as:
\($$C_2H_4 + H_2 → C_2H_6$$$$\Delta H = H_f(C_2H_6) - [H_f(C_2H_4) + H_f(H_2)]$$$$\Delta H = -84.68 - [52.47 + 0]$$$$\Delta H = -84.68 - 52.47$$$$\Delta H = -137.15 kJ/mol$$.\)
for more such questions on enthalpy
https://brainly.com/question/826577
#SPJ8
indicate whether fluorine of bromine has larger value
Answers
Bromine has larger ionic radius and atomic radius than fluorine.
Fluorine has larger ionisation energy and electronegativity than bromine.
What are halogen?Halogen are those elements that are found in group 7 of the periodic table.
Fluorine is in period two of the periodic table, meaning that it contains electrons in only the first two energy levels at ground state. It has a higher ionization energy and electronegativity than bromine.
Learn more about ionic radius here:
https://brainly.com/question/8137711
#SPJ1
Indicate whether fluorine or bromine has a larger value for each of the following properties. a. electronegativity b. ionic radius c. atomic radius d. ionization energy
How can knowledge of separating mixtures help you in daily life and within society? Answer below.
Answers
Answer:
I can say that knowledge of separating mixtures can help us in daily life and within society in the following ways:
1. Purification of water: Separation techniques are used to purify water for drinking and industrial purposes. It is essential to remove impurities from water to prevent diseases.
2. Recycling: Separation techniques are used to separate materials for recycling. This helps reduce the amount of waste in landfills and helps conserve resources.
3. Food industry: Separation techniques are used in the food industry to separate unwanted particles from food products. This helps ensure that the food we eat is safe and free from contaminants.
4. Medicine: Separation techniques are used in the pharmaceutical industry to separate and purify chemicals for use in medicine. This helps ensure that medicines are safe and effective.
5. Environmental protection: Separation techniques are used to remove pollutants from the environment. This helps protect our natural resources and prevent pollution-related health problems.
6. Oil and gas industry: Separation techniques are used to separate crude oil and natural gas into their various components. This helps in the production of energy and other useful products.
In summary, knowledge of separating mixtures is essential in our daily lives and within society. It helps ensure that we have access to safe and clean water, food, medicine, and energy, and also helps protect the environment.
Explanation:
2. When dinitrogen pentoxide is heated, it decomposes to
nitrogen dioxide and oxygen. How many moles of nitrogen
dioxide can be formed from the decomposition of 1.25 g of
dinitrogen pentoxide?
Answers
0.02314 moles of NO₂ can be formed from the decomposition of 1.25 g of dinitrogen pentoxide.
The balanced equation for the decomposition of dinitrogen pentoxide is:
2 N₂O₅ → 4 NO₂ + O₂
The molar mass of N₂O₅ is 108.01 g/mol.
To determine the number of moles of N₂O₅ present in 1.25 g, we use the following calculation:
moles N₂O₅ = mass / molar mass
moles N₂O₅ = 1.25 g / 108.01 g/mol
moles N₂O₅ = 0.01157 mol
From the balanced equation, we can see that 2 moles of N₂O₅ decompose to form 4 moles of NO2. Therefore, the number of moles of NO2 produced can be calculated as:
moles NO₂ = (0.01157 mol N2O5) × (4 mol NO2 / 2 mol N2O5)
moles NO₂ = 0.02314 mol
Therefore, 0.02314 moles of NO₂ can be formed from the decomposition of 1.25 g of dinitrogen pentoxide.
learn more about moles here
https://brainly.com/question/29367909
#SPJ1
1. Calculate the quantity of glucose needed to prepare (a) 250 mL of a 100 mM of
solution (in water), (b) 100 mL of 0.05% solution and (c) 500 mL of 50 mg/mL.
[Formula: C6H12O6 Melting point: 146°C, IUPAC ID: D-glucose, Density:
1.56 g/cm, Molar mass: 180.156 g/mol).
Answers
Answer:
a. mass of glucose required = 4.50 g
b. mass of glucose required = 0.05 g
c. mass of glucose required = 25g
Explanation:
1a. molar mass of glucose = 180.156 g/mol
volume of solution = 250 mL or 250 mL * 1 L/1000 mL = 0.25 L
concentration of solution = 100 mM or 100mM * 1 M/1000 mM = 0.10 M
amount in moles = mass/ molar mass = concentration * volume
mass in grams = concentration * volume * molar mass
mass = 0.10 * 0.25 * 180.156
mass of glucose required = 4.50 g
b. mass concentration of 0.05 % solution = 0.05 g/100 ml = 0.5 g/L
volume of solution = 100 mL = 0.1 L
mass in grams = mass concentration * volume
mass in grams = 0.5 g/L * 0.1 L
mass of glucose required = 0.05 g
c. mass concentration of solution = 50 mg/ mL = 0.05 g/ 0.001 L = 50 g/L
volume of solution = 500 mL = 0.5 L
mass of solute required = concentration * volume
mass required = 50 g/L * 0.5 L
mass of glucose required = 25g
1. Determine the electron-domain geometry and molecular geometry for each of the following.
a. SBr2
b. PI4+
c. IBr2−
2. The following three compounds all have the same general formula, XF4. Compare the electron-domain geometries of these three compounds and explain what characteristic(s) of the central atom causes differences in geometry.
a. SiF4:
b. SeF4:
c. XeF4:
Answers
Answer:
SBr2: Electron geometry-tetrahedral; molecular geometry-trigonal pyramidal
PI4+: Electron geometry-tetrahedral; molecular geometry-tetrahedral
IBr2−: Electron geometry- Trigonal bipyramidal ; molecular geometry- linear
Explanation:
In SBr2, the molecule is of the structure type AX2E2 hence it is based on a tetrahedron but have two lone pairs of electrons hence the molecular geometry is trigonal pyramidal.
PI4+ has four electron domains and all of them are bond pairs hence both electron geometry and molecular geometry are both tetrahedral.
IBr2- is of the structure type AX2E3 hence it is based on a trigonal bipyramd and has a linear molecular geometry.
2)
SiF4 has a tetrahedral molecular and electron domain geometry because the central atom(Si) has no lone pairs.
SeF4 has a trigonal bipyramidal electron geometry with the structure AX4E. Its molecular geometry is See-saw since it has one lone pair of electrons that causes a deviation from its ideal trigonal bypyramidal geometry.
XeF4 has an octahedral electron domain geometry and the molecule is AX4E2. The two lone pairs are positioned above and below the plane of a square hence the molecule is square planar.
what is one advantage of doing a feild experiment instead of a laboratory experiment
Answers
Answer:
Field experiments can often have the potential to give scientists opportunities that are not possible in a lab setting.
Explanation:
Having people "act natural" in a lab setting is impossible to truly achieve, as we all know what happens to our mental state and behavior when we are being actively observed. This is also especially helpful when conducting wildlife research, as there are a myriad of different things influencing animal and plant behavior that would be nigh impossible to recreate perfectly in a controlled lab setting.
Field research can have its disadvantages as well, as it is very hard to only have one thing change (make an independent variable) in a wild environment with ever-changing weather, social effects, etc. Also, you, as the researcher, as causing an impact on the very location that you are observing, which can alter your results in unpredictable ways.
The thing to remember is that each type of study has its advantages and disadvantages; if they didn't, then we'd all do the same type of research! You have to weigh your research options and figure out which one works best for your situation :)
What is the empirical formula for ribose (C5H10Os)?
Answers
The molecular formula for ribose is C5H10O5, so we can find the empirical formula as follows:
The greatest common factor of 5, 10, and 5 is 5.
Dividing the number of carbon atoms (5) by 5 gives 1.
Dividing the number of hydrogen atoms (10) by 5 gives 2.
Dividing the number of oxygen atoms (5) by 5 gives 1.
Therefore, the empirical formula for ribose is CH2O. This means that ribose contains one carbon atom, two hydrogen atoms, and one oxygen atom in its simplest ratio.
How many moles of KBr are dissolved in 60.2 mL of a 3.50 M solution?
Answers
There are 0.2107 moles of KBr are dissolved in 60.2 mL of a 3.50 M solution.
The molarity of a substance is defined as the number of moles of solute present in 1 litre of a solution.
According to the given data, the molarity of the solution tells us that there are 3.50 moles of KBr in 1000mL of solution. But we only have 60.2mL of solution, so with a mathematical rule of three we can calculate the amount of moles in 60.2mL:
1000 ml - 3.50 moles
60.2 ml -x = 60.2 ml× 3.50 moles/1000 ml
x= 60.2 ml -0.2107 moles
So, there are 0.2107 moles of KBr.
To know more about moles here
https://brainly.com/question/29293653
#SPJ1
Which of these statements best explains why chemistry is reliable?
Answers
Answer:
It gives the same result when an experiment is repeated.
Explanation:
Below are the possible answers to the question:
It is biased.
It cannot be verified.
It cannot add new evidence to existing evidence.
It gives the same result when an experiment is repeated.
The correct answer would be that it gives the same result when an experiment is repeated.
If a reaction is conducted in chemistry and certain results are obtained, once a detailed procedure of the experiment is known along with all the chemicals involved, such reaction/experiment can be repeated anywhere in the world and the same result would be obtained.
The repeatability of experiments always makes the experiments to be reliable. Hence, chemistry is reliable because it gives the same result without any variation when experiments are repeated under similar conditions.
What is the [OH-] in a solution that has a pOH = 2.28
Show explanations please.
Answers
Answer:
The value of pOH of a solution is 2.28
We need to find the [OH-] in a solution. We know that,
pOH=-\text{log}[OH^-]pOH=−log[OH−]
Put all the values,
\begin{gathered}2.28=-\log[OH^-]\\\\=0.00524\end{gathered}2.28=−log[OH−]=0.00524
So
What do the blue and red lines indicate?
A cold front is moving to the north and west before a warm front moves through.
A cold front is moving to the south and east before a warm front moves through.
A warm front is moving to the north and east before a cold front moves through.
A warm front is moving to the south and west before a cold front moves through.
WILL GIVE BRAINLIEST!

Answers
Answer:
this the one i needed help on pleasee sb help meh out
Explanation:
Answer:
A cold front is moving to the north and west before a warm front moves through.
Explanation:
How many grams of Oxygen are in 4 moles of C2H4O2? Show work.
Answers
The mass of oxygen in 4 moles of C2H4O2 is 128g.
How to calculate mass?The mass of a substance can be calculated by multiplying the number of moles of the substance by its molar mass.
Molar mass of C2H4O2 = 12(2) + 1(4) + 16(2) = 60g/mol
mass of C2H4O2 = 60g/mol × 4 moles = 240grams
However, the mass of oxygen in the compound can be calculated as follows:
molar mass of oxygen/molar mass of compound × 240 grams
32g/mol ÷ 60g/mol × 240g
= 128g
Therefore, the mass of oxygen in 4 moles of C2H4O2 is 128g.
Learn more about mass at: https://brainly.com/question/11954533
#SPJ1
A scientific hypothesis is
ANSWER:
predictive.
testable.
explanatory.
all of the above.
Answers
Answer:
All of the above.
Explanation:
For a scientific hypothesis to be considered a hypothesis, it has to be testable. When conducting a lab experiment, it also allows the tester to predict what might occur during and after the experimentation. They are also explanatory. For example, theories are hypotheses that have been verified and can explain why something in nature takes place.
Which element is in the same group as Al (aluminum)?
O A. B (boron)
O B. Si (silicon)
O C. Zn (zinc)
O D. Mg (magnesium)
Answers
Which daughter element is produced from the alpha decay of 213 over 85 At ?
A. 213 over 86 Rn
B.217 over 87 Fr
C. 213 over 84 Po
D. 209 over 83 Bi

Answers
Answer:
209
83 Bi
Explanation:
213 213 - 4 4
85 At = 85 - 2 Y + 2 He


What is the average atomic mass of 10 hydrogen -1 molecules?
Answers
Answer:
1.674 x 10^-23 grams
Explanation:
Hydrogen-1 is called Protium
wikipedia
atomic mass of Protium is 1.00794 amu
sciencedirectcom
atomic mass of 10 Protiums is 10.0794 amu
10.0794 amu in grams is
1.6737236x10^-23 grams
mole practice chemistry
show your work

Answers
The number of moles in 44 g of CO₂ is one mole. Number of moles of 98 g of O₂ is 3 moles. Similarly the number of moles in 12 g of MgCl₂ is 0.12.
What is one mole ?Any substance that contains 6.02 × 10²³ atoms or molecules is called one mole of that substance. This number is called Avogadro number. One mole of every element contains Avogadro number of atoms.
The mass of one mole of a compound is called its molar mass. We can determine the number of moles in a given mass of the compound by dividing the mass by molar mass.
no.of moles = given mass/molar mass.
Molar mass of CO₂ = 44 g
hence 44 g of is one mole.
Molecular mass of O₂ = 32 g
moles in 98 g = 98/32 = 3 moles.
Molar mass of MgCl₂ = 95 g/mol
moles in 12 g = 12 /95 = 0.12 moles.
In the similar way, the number of moles of all compounds for the given mass can be calculates.
Find more on molar mass :
http://brainly.com/question/13152455
#SPJ1
Given 0.08 of KMnO4, calculate the number of molecules
Answers
The number of molecules in the permanganate is 4.8 * 10^22 molecules
What is the number of the molecules?We know that if we are to obtain the number of molecules form the number of the moles of the substances then as a matter of necessity we would have to turn to the Avogadro's law and that is what we are going to do here.
We have that;
If 1 mole contains about 6.02 * 10^23 molecules
0.08 moles would contain 0.08 * 6.02 * 10^23/ 1
= 4.8 * 10^22 molecules
Hence, we have about 4.8 * 10^22 molecules in the permanganate
Learn more about molecules:https://brainly.com/question/19922822
#SPJ1
Q2) Circle and name the two types of oxygen containing functional groups on each sugar.
If a group occurs multiple times, only circle and name it once per sugar.

Answers
Disaccharides are sugars made up of two saccharides units linked together by a COC bond.
What is the purpose of a disaccharide?Like any other carbohydrate, disaccharides serve as the body's energy source. Disaccharides are converted by our bodies into simple sugars (simple sugars) for the small intestine to absorb when we ingest meals containing them.
What does it mean to be a disaccharide?Disaccharide, commonly known as double sugar, is any material made up of two simple sugar molecules (monosaccharides) joined together.
To know more about disaccharides visit:
https://brainly.com/question/16580858
#SPJ1
Scientists found the remains of dinosaur fossils in a remote section in the west, located
within ten miles of a meteor crash site. Which statement can be made about the
dinosaur and the meteor?
O a. The dinosaur could have died from a flood.
O b. The dinosaur and the meteor are not related.
O c. The dinosaur could have died from freezing to death.
O d. The dinosaur could have died due to an meteor crash.
Answers
Answer:
D The dinosaur could have died due to an meteor crash.
Explanation:
Answer:
D
Explanation:
The dinosaurs fossils were found ten miles away from the meteor crash site. Correct me if I'm wrong
Nitrogen and hydrogen combine at a high temperature, in the presence of a catalyst, to produce ammonia.
N2(g)+3H2(g)⟶2NH3(g)
Assume 0.290 mol N2 and 0.954 mol H2 are present initially.
After complete reaction, how many moles of ammonia are produced?
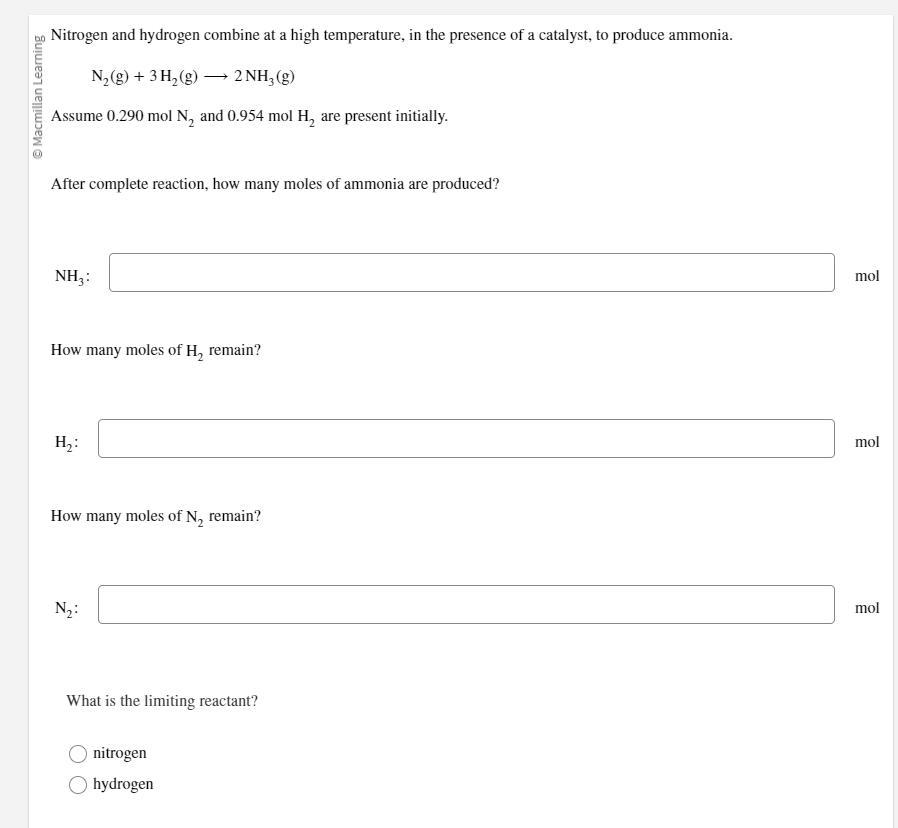
Answers
Answer:
17 reactions
Explanation:
(c) 45 g C,H, react with 45 g Cl₂ according to the equation:
Cl₂ + C6H6 C6H5Cl + HCI. What is the limiting reactant? What mass of HCI will be produced?
-
Answers
In the given reaction, the limiting reactant is C₆H₆ (benzene).
To determine the limiting reactant as well as calculate the mass of HCl produced, compare the moles of each reactant.
The number of moles for each reactant:
Molar mass of Cl₂ = 35.5 g/mol + 35.5 g/mol = 71 g/mol
Moles of Cl₂ = mass of Cl₂ / molar mass of Cl₂
= 45 g / 71 g/mol
= 0.634 moles of Cl₂
Molar mass of C₆H₆ (benzene) = 12 g/mol + 6(1 g/mol) = 78 g/mol
Moles of C₆H₆ = mass of C₆H₆ / molar mass of C₆H₆
= 45 g / 78 g/mol = 0.577 moles of C₆H₆
Determine the stoichiometry between Cl₂ and HCl:
Cl₂ + C₆H₆ → C₆H₅Cl + HCl
Here, we can see that 1 mole of Cl₂ produces 1 mole of HCl.
Thus, the limiting reactant is C₆H₆ (benzene).
Calculate the mass of HCl produced:
Molar mass of HCl = 1 g/mol + 35.5 g/mol = 36.5 g/mol
Moles of HCl produced = moles of C₆H₆ = 0.577 moles
Mass of HCl produced = moles of HCl produced × molar mass of HCl
Mass of HCl produced = 0.577 moles × 36.5 g/mol
≈ 21.04 g
Therefore, approximately 21.04 grams of HCl will be produced.
For more details regarding limiting reactant, visit:
https://brainly.com/question/10090573
#SPJ1
Complete the fourth column of the table.Express your answer using two significant figure

Answers
Using Avogadro's Law, we have
\(\frac{V_1}{n_1}=\frac{V_2}{n_2}\)In the fourth column, we have to find n2.
\(n_2=\frac{V_2\cdot n_1}{V_1}\)Where V1 = 8.66 L, V2 = 10.9 L, and n1 = 0.0021 mol.
\(n_2=\frac{10.9L\cdot0.0021\text{mol}}{8.66L}=0.0026mol\)Therefore, n2 = 0.0026 moles.
How much energy (in J) is lost when a sample of iron with a mass of 28.3 g cools from 66.0 degrees celsius to 24.0 degrees celsius.
Answers
Answer:
Q = -535 J.
Explanation:
Hello there!
In this case, according to the given information, it turns out possible for us to calculate the lost energy according to the following and generic heat equation:
\(Q=mC(T_F-T_i)\)
Thus, since the specific heat of iron is 0.450 in the SI units, we can plug in the mass and temperatures to obtain:
\(Q=28.3g*0.450\frac{J}{g\°C} (24.0\°C-66.0\°C)\\\\Q=-535J\)
Regards
Which substance is solvent and which substance is solute (number 4)

Answers
Answer:
Solvent - water
solute - lemonade powder
Explanation:
You should learn the definitions of al them words. I made them notes in grade 7 dont judge and was bored while studying so i highlited everything and i thought it looked cute back then. We all did that.

Which three of the following statements accurately describe the blood buffering system in humans? The blood buffering system...
a) utilizes the H2CO3/HCO3^- conjugate acid/base pair.
b) maintains the pH of blood near 7.4.
c) regulates the blood pH at 7.4 +/- one pH unit.
d) depends on the ionization of H2PO4^-.
e) utilizes the acetic acid/acetate conjugate acid/base pair.
f) is facilitated by the enzyme carbonic anhydrase, which interconverts carbon dioxide and water to carbonic acid (ionizes into bicarbonate and H^+).
Answers
Answer:
b) maintains the pH of blood near 7.4.
c) regulates the blood pH at 7.4 +/- one pH unit.
f) is facilitated by the enzyme carbonic anhydrase, which interconverts carbon dioxide and water to carbonic acid (ionizes into bicarbonate and H^+).
Explanation:
The pH of human blood is slightly acidic i. e. 7.4. The enzyme carbonic anhydrase is responsible for the regulation to neutral and prevent it from acidic. the enzyme carbonic anhydrase helps in the conversion of carbon dioxide to carbonic acid and bicarbonate ions. When the blood reach to lungs, the bicarbonate ions convert back to CO2 and this carbondioxide is exhaled from the body.
Chemistry problems
1. 1.5 moles of potassium sulfate (K SO4) were dissolved in 1000 grams of water (H2O). Find the % and Cm.
2. 10 grams of sulfuric acid (H2SO4) was added to 500 ml of 10% solution of potassium hydroxide (KOH) with a density of 1.1 g/ml. Find the mass of potassium sulfate (K SO4) formed.
3. Find the mass of the salt formed by the reaction of 7.3 grams of hydrochloric acid (HCl) with 5.6 liters (5600 ml) of ammonia (NH3).
4. Find the volume of hydrogen gas (H2) produced by the reaction of 13 grams of zinc with a solution containing 30 grams of sulfuric acid (H2SO4).
5. How much of the concentrated original solution (70%) of acetic acid is needed to prepare 500 grams of 3% (percentage solution)?
Answers
1. The % concentration is 20.7% and the molar concentration, Cm, is 1.5 M.
2. 7.8 grams of potassium sulfate will be formed.
3. 10.7 grams of ammonium chloride will be formed.
4. The volume of hydrogen gas that will be produced is 3.86 liters.
5. 21.43 grams of the 70% acetic acid is needed to prepare 500 grams of 3% acetic acid solution.
What is the percentage concentration?1. Mass of potassium sulfate = 1.5 moles * (174.26 g/mol) = 261.39 g
Mass of water (H₂O) = 1000 g
% = (mass of solute/mass of solution) x 100
% = (261.39 g / (261.39 g + 1000 g)) x 100
% ≈ 20.7%
Cm = moles of solute / volume of solution
Moles of potassium sulfate (K2SO4) = 1.5 moles
Volume of water (H2O) = 1000 g / (density of water) = 1000 g / 1 g/mL = 1000 mL = 1 L
Cm = 1.5 moles / 1 L
Cm = 1.5 M
2. The balanced equation for the reaction is:
H₂SO₄ + 2 KOH → K₂SO₄ + 2 H₂O
Molar mass of sulfuric acid (H₂SO₄) = 98.09 g/mol
Moles of sulfuric acid = 10 g / 98.09 g/mol
Moles of sulfuric acid = 0.102 mol
Based on the mole ratio of the reaction, 0.102 moles of sulfuric acid will react to form 0.102 moles of potassium sulfate.
Molar mass of potassium sulfate = 174.26 g/mol
Mass of potassium sulfate = 0.102 mol x 174.26 g/mol
Mass of potassium sulfate ≈ 17.8 g
3. The balanced equation for the reaction is:
HCl + NH₃ → NH₄ClMolar mass of hydrochloric acid (HCl) = 36.46 g/mol
Moles of hydrochloric acid (HCl) = 7.3 g / 36.46 g/mol
Moles of hydrochloric acid ≈ 0.2 mol
Based on the mole ratio of the reaction, 0.2 moles of hydrochloric acid will react to form 0.2 moles of ammonium chloride.
Molar mass of ammonium chloride (NH₄Cl) = 53.49 g/mol
Mass of ammonium chloride = 0.2 mol x 53.49 g/mol
Mass of ammonium chloride ≈ 10.7 g
4. The balanced equation for the reaction is:
Zn + H₂SO₄ → ZnSO₄ + H₂Molar mass of zinc (Zn) = 65.38 g/mol
Moles of zinc = 13 g / 65.38 g/mol
Moles of zinc ≈ 0.199 mol
Based on the mole ratio of the reaction, 0.199 moles of zinc will react to produce 0.199 moles of hydrogen gas.
Volume of sulfuric acid = 30 g / (density of H₂SO₄ )
The density of H₂SO₄ is 1.84 g/mL
Volume of sulfuric acid = 30 g / 1.84 g/mL
Volume of sulfuric acid ≈ 16.3 mL or 0.0163 L
Using the ideal gas law, the volume of hydrogen gas produced will be:
V = nRT / P
V = (0.199 mol)(0.0821 L·atm/(mol·K))(273 K) / (1 atm)
V ≈ 3.86 L
5. Assuming that the concentrated original solution of acetic acid is 100% acetic acid (CH₃COOH).
Mass of acetic acid = 500 g x (3/100) = 15 g
The concentrated original solution, however, is 70% acetic acid.
70% acetic acid (mass) = 100% acetic acid (unknown mass)
0.7 * (unknown mass) = 15 g
Solving for the unknown mass:
unknown mass = 15 g / 0.7
unknown mass ≈ 21.43 g
Learn more about percentage concentration at: https://brainly.com/question/18761928
#SPJ1
11. The pH values of some solutions are given below pH 14.0 1.0 L 8.0 N 6.5 n P 7.0 Solution M Z (a) Identify the solution with the lowest concentration of hydrogen ion. Give reason for your (1mk) answer
Answers
Answer: 14.0
Explanation: 14.0 is a base. The more basic, the less hydrogen ion concentration.
Magnesium hydroxide reacts with chlorine to form magnesium chloride,
magnesium chlorate and water. How many grams of magnesium hydroxide is
needed to yield 8.00 moles of magnesium chlorate?
77.8 g Mg(OH)2
9178.1 g Mg(OH)2
2799.6 g Mg(OH)2
.823 g Mg(OH)2
How many grams of sodium sulfato pro
Answers
The grams of magnesium hydroxide needed to yield 8.00 moles of magnesium chlorate is approximately 466.64 g. None of the options provided match the calculated value of 466.64 g.
To determine the grams of magnesium hydroxide (Mg(OH)2) needed to yield 8.00 moles of magnesium chlorate (Mg(ClO3)2), we need to consider the balanced chemical equation for the reaction between magnesium hydroxide and chlorine.
The balanced equation is as follows:
2 Mg(OH)2 + 6 Cl2 → 2 Mg(ClO3)2 + 2 H2O
From the balanced equation, we can see that 2 moles of Mg(OH)2 react with 6 moles of Cl2 to produce 2 moles of Mg(ClO3)2.
Therefore, the stoichiometric ratio is 2 moles of Mg(OH)2 : 2 moles of Mg(ClO3)2.
To calculate the grams of Mg(OH)2 needed, we can use the stoichiometric ratio and the given moles of Mg(ClO3)2.
Given:
Moles of Mg(ClO3)2 = 8.00 moles
Using the stoichiometric ratio, we have:
8.00 moles Mg(ClO3)2 × (2 moles Mg(OH)2 / 2 moles Mg(ClO3)2) = 8.00 moles Mg(OH)2
To convert moles to grams, we need to multiply by the molar mass of Mg(OH)2.
The molar mass of Mg(OH)2 = (24.31 g/mol) + (2 * 16.00 g/mol) = 58.33 g/mol
Grams of Mg(OH)2 = 8.00 moles Mg(OH)2 × 58.33 g/mol = 466.64 g
Therefore, the grams of magnesium hydroxide needed to yield 8.00 moles of magnesium chlorate is approximately 466.64 g.
For more such questions on magnesium chlorate
https://brainly.com/question/12358640
#SPJ11