Answers
Answer: H2O has 8 electrons, GeBr4 has 32 electrons, and RbHO has 37 electrons.
Hope this helped! :)
Related Questions
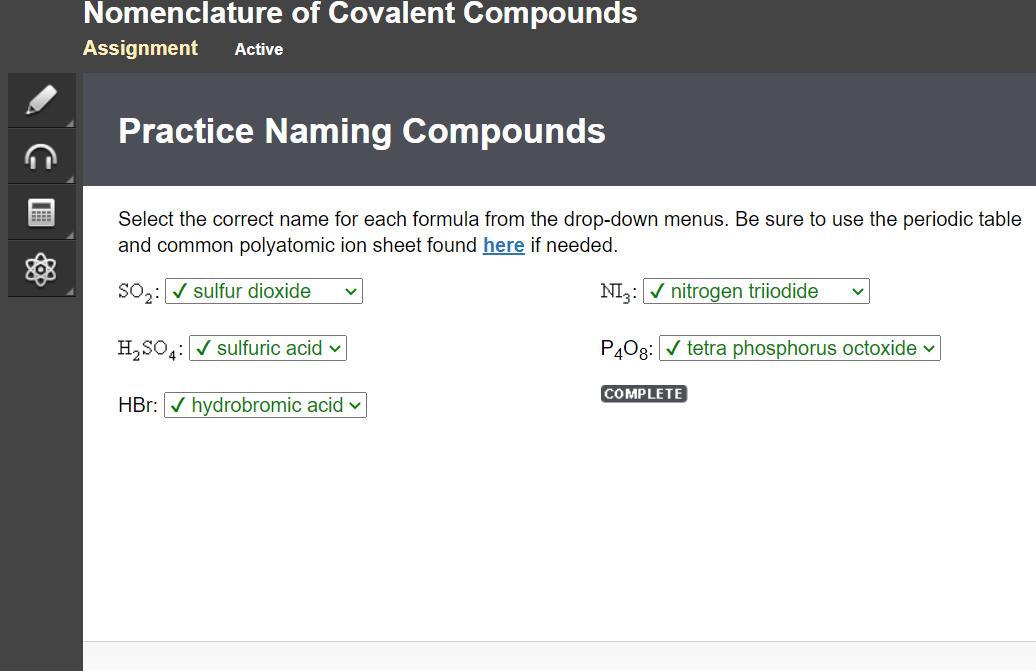
Select the correct name for each formula from the drop-down menus. Be sure to use the periodic table
and common polyatomic ion sheet found here if needed.
Answers
Answer:
here are the answers edge2020
Explanation:

Answer:
Here is 2/5 and the next slide answers too!
Explanation:
Hope it helps!!

for permanganate test, you will use solution of kmno4 in sulfuric acid. how much of kmno4 (in g) and how much of sulfuric acid (in ml) you would have to take to prepare 100 ml solution in which concentrations of kmno4 is 0.5 m and concentrations of h2so4 is 1 m. concentration of commercially available sulfuric acid is 18 m.
Answers
To prepare 100 ml of a solution with 0.5 M KMnO4 and 1 M H2SO4, we need to calculate the amount of each compound needed.
For KMnO4:
0.5 M = 0.5 mol/L
0.5 mol/L × 0.1 L = 0.05 mol
0.05 mol × 158.034 g/mol = 7.9017 g
Therefore, we need 7.9017 g of KMnO4 to prepare the solution.
For H2SO4:
1 M = 1 mol/L
1 mol/L × 0.1 L = 0.1 mol
0.1 mol ÷ 18 M = 0.00556 L = 5.56 ml
Therefore, we need 5.56 ml of 18 M H2SO4 to prepare the solution.
In conclusion, to prepare 100 ml of a solution with 0.5 M KMnO4 and 1 M H2SO4, we need 7.9017 g of KMnO4 and 5.56 ml of 18 M H2SO4.
To know more about permanganate test refer here:
https://brainly.com/question/30087623
#SPJ11
When a white powder of sodium chloride is dissolved in water a colourless solution is formed.on the other hand,when a white powder of copper (ii) sulphate is dissolved in water a blue solution is formed explain why the copper (ii) sulphate is blue
Answers
Colour can be changed because of the change in the ligands. Some complex exhibit colour mainly due to the crystal field splitting. Here copper (ii) sulphate is known as blue vitriol.
In hydrated copper sulfate, four water molecules are present as ligands. In the presence of these ligands, d-orbital are no longer degenerate in energy. Hence, d-d transition takes place absorbing red wavelength. The complementary colour blue is reflected.
When concentrated HCl is added, the water ligands are replaced by chloride ligands. This forms copper chloride complex. The d-d transition takes place by absorbing violet wavelength. So yellow colour is reflected.
To know more about crystal field splitting, visit;
https://brainly.com/question/31667042
#SPJ1
Which of these is an example of a biotic factor?
hot desert sun
Mojave River
acrid desert air
Mojave grasshoppers
Answers
Answer: Mojave grasshopper
Explanation: this is the answer because it is living
A biotic factor is something that it living. An abiotic factor is something that is nonliving. The only object that is living in the choices is the grasshopper. :)
What is an empirical formula?
Answers
Answer:
a formula giving the proportions of the elements present in a compound but not the actual numbers or arrangement of atoms.
Hope this helped!!!
Answer:
Empirical formulas show the simplest whole-number ratio of atoms in a compound
Explanation:
what is mean by vector
Answers
Answer:
A quantity having direction as well as magnitude is called vector.
How would you change the distance between two charged particles to increase the electric force between them by a factor of 25?
Answers
Answer:
reduce the distance by the factor of 5
chris needs to mix a 10% acid solution with a 30% acid solution to create 100 milliliters of a 16% solution. how many milliliters of each solution must chris use?
Answers
Let's assume that Chris uses x milliliters of the 10% acid solution and (100 - x) milliliters of the 30% acid solution. Hence this assumption results in 16% solution of 100 milliliters.
To find the amount of acid in the resulting 16% solution, we can use the following equation:
0.1x + 0.3(100 - x) = 0.16(100)
We have got 0.16(100) and after Simplifying the equation, we will get:
0.1x + 30 - 0.3x = 16
-0.2x = -14
And the final solution would be after doing the above equation is
x = 70
Therefore, Chris needs to use 70 milliliters of the 10% acid solution and (100 - 70) = 30 milliliters of the 30% acid solution to create 100 milliliters of a 16% solution.
Learn more about Acid solutions here:
brainly.com/question/13208021
#SPJ4
The reaction of 2-phosphoglycerate to phosphoenolpyruvate is a ____________ reaction.
Answers
Hi there,
I hope you and your family are staying safe and healthy!
The answer is: Enolase
Enolase is used to convert 2-phosphoglycerate (2PG) to phosphoenolpyruvate (PEP).
Please leave 5 stars and a like if you find this answer helpful.
Happy to help!
~Garebear
The pressure of a gas sample at 60.0 °C is decreased from 3.40 atm to 2.60 atm by cooling at a constant volume. What is the temperature (in °C) of the gas at 2.60 atm?
Answers
When the pressure of a gas is decreased from 3.40 atm to 2.60 atm, the temperature of the gas will decrease from 60.0 degree celsius to 45.88 degree celsius.
What is Gay-Lussac law?Gay -Lussac law states that the pressure and temperature is in direct proportions. Thus, pressure increases with an increase in temperature. The relation can be mathematically expressed as follows:
P/T = a constant. Hence,
P1/T1 = P2/T2
The initial pressure of the sample is given, 3.46 atm and the initial temperature is 60 °C. The final temperature of the gas when gas the expand to the lower pressure of 2.60 atm is calculated as follows:
\(T_{2} = \frac{P_{2}T_{1}}{P_{1}}\)
= (60 °C× 2.60 atm) / 3.40 atm
= 45.88 °C.
Hence, the final temperature of the gas which is expanded from the pressure of 3.40 atm to 2.60 atm with an initial temperature of 60 °C is 45.88 °C.
To find more about Gay-Lussac law, refer the link below:
https://brainly.com/question/2683502
#SPJ2
True or false; A solution always contains only one solvent.
Answers
A solution is defined as a mixture of two or more substances, usually, a solute and a solvent, and the difference between these two are in quantity, solute represents the smallest amount and solvent will represent the highest amount, and while you can have more than one solute, you can only have one solvent for a solution. Therefore the statement is true
Write the formula of the coordination compound lithium pentacyanocobaltate(II). and Write the formula of the coordination compound pentaamminechlorocobalt(III) nitrate. Enclose the coordination complex in square brackets, even if there are no counter ions. Do not enclose a ligand in parentheses if it appears only once. Enter water as H2O
Answers
The formula of the coordination compound lithium pentacyanocobaltate(II) is [LiCo(CN)₅]²⁻.
What is lithium pentacyanocobaltate(II) ?
Pentacyanocobaltate attracted attention as an early example of a metal complex that reacts with hydrogen. It contains low-spin cobalt(II) with a doublet ground state.
Let's break down the formula to understand its components:
The central metal ion is cobalt (Co) in the +2 oxidation state, denoted as Co(II).
The ligands are pentacyanocobaltate, which means there are five cyanide ligands (CN⁻) bonded to the cobalt ion.
The coordination complex is negatively charged, with a charge of 2-, so it requires two lithium ions (Li⁺) as counter ions to balance the charge.
Therefore, the complete formula is [LiCo(CN)₅]²⁻, where the square brackets indicate the coordination complex and the superscript 2- indicates the charge.
The formula of the coordination compound pentaamminechlorocobalt(III) nitrate is [Co(NH₃)₅Cl]NO₃.
Breaking down the formula:
The central metal ion is cobalt (Co) in the +3 oxidation state, denoted as Co(III).
The ligands are pentaamminechlorocobalt, indicating there are five ammonia ligands (NH₃) and one chloride ligand (Cl⁻) bonded to the cobalt ion.
There is no need for any counter ions to balance the charge in this compound.
Thus, the complete formula is [Co(NH₃)₅Cl]NO₃, where the square brackets denote the coordination complex, and NO₃ indicates the presence of nitrate ions as the counter ions.
To learn more about lithium pentacyanocobaltate(II) from the given link
https://brainly.com/question/30766130
#SPJ4
how many moles of LiNO3 are in 250mL of a 0.30M solution?
Answers
\(LiNO_{3}\)There are 0.075 moles of \(LiNO_{3}\) in 250 mL of a 0.30 M solution.
To determine the number of moles of \(LiNO_{3}\) in 250 mL of a 0.30 M solution, we can use the formula:
moles of solute = concentration (M) x volume (L)
First, we need to convert the volume from milliliters (mL) to liters (L):
250 mL = 0.25 L
Next, we can substitute the given values into the formula:
moles of \(LiNO_{3}\) = 0.30 M x 0.25 L
moles of \(LiNO_{3}\) = 0.075 mol
Therefore, there are 0.075 moles of \(LiNO_{3}\) in 250 mL of a 0.30 M solution.
Learn more about moles, here:
https://brainly.com/question/15209553
#SPJ1
If a solution is made by using 0.45 moles of NaCl and dissolving it with water so that the total volume is 0.750 liters, what will the concentration of that solution be in molarity?
Answers
Answer:
.6 M
Explanation:
.45 moles / .750 liters = .6 M
describe un modelo matemático para representar lo que sucede en cada una de las reacciones químicas (incluye toda la simbología que puedas) y clasifícalas según consideres de acuerdo con lo abordado en el tema.
1. El hidrógeno molecular reacciona con el oxígeno molecular y produce agua
2. El Óxido de calcio reacciona con el agua y produce hidróxido de calcio
3. El sulfuro de hierro (II) se produce cuando reacciona el azufre y el hierro en su forma atómica
4. El ácido sulfuroso se descompone por la acción de calor en dióxido de azufre gaseoso y agua
5. El carbonato de calcio se descompone por la acción de calor en oxido de calcio y dióxido de carbono gaseoso.
6. El magnesio reacciona con el ácido clorhídrico y produce dióxido de magnesio e hidrógeno gaseoso.
AYUDAAAAA
Answers
Responder:
2H2 + O2 → 2H2O
CaO + H2O → Ca (OH) 2
Fe + S → FeS
H2SO3 → SO2 + H2O
CaCO3 → CaO + CO2
Explicación:
2H2 + O2 → 2H2O
2 moléculas de hidrógeno gaseoso reaccionan con oxigente para producir 2 moléculas de agua
CaO + H2O → Ca (OH) 2
El óxido de calcio reacciona con el agua para producir hidróxido de calcio.
Fe + S → FeS
El hierro reacciona con el azufre para producir sulfuro de hierro.
H2SO3 → SO2 + H2O
Por descomposición, el ácido sulfuroso se descompone para producir dióxido de azufre y agua.
CaCO3 → CaO + CO2
El carbonato de calcio se descompone para producir óxido de calcio y dióxido de carbono.
Given the following reactions:
CaCO3 (s) -> CaO (s) + CO2 (g) H = 178.1
C (s, graphite) + O2 (g) -> CO2 (g) H = -393.5 kJ
The enthalpy of the reation CaCO3 (s) -> CaO (s, graphite) + O2 (g) is _______ kJ
Answers
The enthalpy change for the reaction CaCO3 (s) -> CaO (s, graphite) + O2 (g) is 571.6 kJ.
The enthalpy of the reaction CaCO3 (s) -> CaO (s, graphite) + O2 (g) can be calculated by summing the enthalpies of the individual reactions involved. The given information provides the enthalpy change for the decomposition of CaCO3 (s) and the combustion of C (s) to form CO2 (g). By combining these reactions, the enthalpy change for the overall reaction can be determined.
The given reactions are:
CaCO3 (s) -> CaO (s) + CO2 (g) (H = 178.1 kJ)
C (s, graphite) + O2 (g) -> CO2 (g) (H = -393.5 kJ)
To calculate the enthalpy change for the reaction CaCO3 (s) -> CaO (s, graphite) + O2 (g), we need to subtract the enthalpy change of reaction 2 from the enthalpy change of reaction 1. Since the enthalpy change is an extensive property, we can subtract the enthalpies directly:
ΔH = H(reaction 1) - H(reaction 2)
= 178.1 kJ - (-393.5 kJ)
= 178.1 kJ + 393.5 kJ
= 571.6 kJ
Therefore, the enthalpy change for the reaction CaCO3 (s) -> CaO (s, graphite) + O2 (g) is 571.6 kJ.
To learn more about enthalpy click here:
brainly.com/question/32882904
#SPJ11
nguyên tố hóa học là tập hợp những nguyên tử có cùng
Answers
Answer:
Please dear user⚠️Stop asking nonsense question for free⚠️Brainly app is an app that helps students from their homeworks⚠️Or you'll get banned⚠️If a 200 kg person stands next to a 100 kg person. Which person would have a larger gravitational attraction?
Answers
Answer:
200 kg
Explanation:
the 200 kg person is heavier due to gravity's force the 100 kg person has less force than the other person
Answer:
massive one that is 200kg
Explanation:
Since the gravitational force is directly proportional to the mass of both interacting objects, more massive objects will attract each other with a greater gravitational force. So as the mass of either object increases, the force of gravitational attraction between them also increases.
A substantial imbalance between demand and response capability under conditions in which failure has important consequences refers to.
Answers
A substantial imbalance between demand and response capability under conditions in which failure has important consequences refers to STRESS. It may affect the functioning of a biological system.
Homeostasis and stressHomeostasis refers to the state of internal (steady) equilibrium, which is required for the normal functioning of a biological system.
Conversely, stress can be defined as any environmental factor (e.g., excessive temperature condition) which is directly affecting the normal functioning of an organism.
Stress may lead to the loss of the homeostatic state when conditions cannot be supported by the organism.
Learn more about stress here:
https://brainly.com/question/11819849
How many molecules are in 1 mole of nitrogen sulfide
Answers
Answer:
46.07
Explanation:
im not 100 sure this is right almost tho
please mark brainliest
Which units express heat capacity? J/°C, J/K, cal/°C, cal/K J/(gi°C), J/(giK), cal/(gi°C), cal/(giK) J, cal °C, K
Answers
Answer:
a
Explanation:
The heat capacity of a substance is the heat energy required to rise its temperature per one degree Celsius. Hence its unit is J/°C.
What is heat capacity ?Heat capacity is the amount of heat energy required to raise the temperature of a substance by 1 degree Celsius or 1 Kelvin. It is expressed in the following units:
Joules per degree Celsius (J/°C)
Joules per Kelvin (J/K)
Calories per degree Celsius (cal/°C)
Calories per Kelvin (cal/K)
Joules per gram per degree Celsius (J/(g·°C))
Joules per gram per Kelvin (J/(g·K)) etc.
If in terms of simply the energy, then, The following units are used.
Joules (J) , Calories (cal) , Degrees Celsius (°C), Kelvin (K)
The choice of unit depends on the specific application and the system of units being used. The SI unit for heat capacity is J/K, while the traditional unit is cal/°C.
The use of per gram units is common in the context of specific heat capacity, which is the amount of heat energy required to raise the temperature of a unit mass of a substance by 1 degree Celsius or 1 Kelvin.
Therefore, here, the unit of heat capacity is J/°C.
Find more on heat capacity :
https://brainly.com/question/28302909
#SPJ7
How many unique gametes could be produced through independent assortment by an individual with the genotype AaBbCCDdEE?a) 4b) 8c) 16d) 32e) 64
Answers
The correct answer is option b) 8.
To determine the number of unique gametes that can be produced through independent assortment, we need to consider the number of possible allele combinations for each gene.
The individual in question has the genotype AaBbCCDdEE, where each letter represents a different gene locus.
The genotype indicates that there are two alleles (A and a) for the first gene, two alleles (B and b) for the second gene, two alleles (C and C) for the third gene, two alleles (D and d) for the fourth gene, and two alleles (E and E) for the fifth gene.
To calculate the number of unique gametes, we multiply the number of possible allele combinations for each gene:
Number of allele combinations for the first gene: 2 (A and a)
Number of allele combinations for the second gene: 2 (B and b)
Number of allele combinations for the third gene: 1 (C)
Number of allele combinations for the fourth gene: 2 (D and d)
Number of allele combinations for the fifth gene: 1 (E)
Total number of unique gametes = 2 × 2 × 1 × 2 × 1 = 8
Therefore, the correct answer is option b) 8. A total of 8 unique gametes could be produced through independent assortment by an individual with the genotype AaBbCCDdEE.
To know more about independent assortment, visit:
https://brainly.com/question/32214951
#SPJ11
SUB TO RahimZ for 15 points i want 100 subs
Answers
2) a chemist combines 122.0 kg of ammonia with 211.4 kg ofcarbon dioxide, and obtains 185.1 kg of urea.a) determine the limiting reactant.b) determine the theoretical yield of urea. (answer: 215.3 kg)c) determine the percent yield for the reaction. (answer: 86.0%)d) how many kg of the excess reactant is left? (answer: 53.5 kg)
Answers
A. Ammonia is the limiting reactant.
B. Theoretical yield of urea is 215.3 kg.
C. Percent yield for the reaction is 86.0%
D. The mass of the excess carbon dioxide left is approximately 54.1 kg.
a) To identify the limiting reactant, we should compare the amount of products formed from each reactant. The chemical equation for the formation of urea \((NH_2CONH_2)\) by combining ammonia \((NH_3)\) and carbon dioxide \((CO_2)\) is as follows:
\(2 NH_3 + CO_2 - > NH_2CONH_2 + H_2O\)
The stoichiometry of the balanced equation indicates that the ratio of ammonia to urea is 2:1.
We can find the number of moles for each reactant using the following masses:
Moles of ammonia = 122.0 kg / 17.03 g/mol = 7.17 mol
Moles of carbon dioxide = 211.4 kg / 44.01 g/mol = 4.80 mol
It takes 14.34 moles of ammonia to react completely with the available carbon dioxide because the ratio of ammonia to urea is 2:1. But the amount of ammonia we have is less than we need - only 7.17 mol. As a result, ammonia is the limiting reactant.
b. Based on the limiting reactant, it is possible to calculate the theoretical yield of urea. We can use the moles of ammonia, which is the limiting reactant, to calculate the moles of urea:
Moles of urea = 7.17 mol / 2 = 3.58 mol
We can determine the theoretical yield of urea using the molar mass of urea (60.06 g/mol) as a starting point:
Theoretical yield of urea = 3.58 mol * 60.06 g/mol = 215.3 kg
C. The actual yield (185.1 kg) is calculated by dividing it by the theoretical yield (215.3 kg), then multiplying the result by 100%.
Percent yield = (185.1 kg / 215.3 kg) * 100% = 86.0%
D. We can calculate the amount of non-limiting reactant that has not reacted yet to determine the excess reactant. Since ammonia is the limiting reactant, we must determine how much excess carbon dioxide there is:
Moles of excess carbon dioxide = Moles of carbon dioxide initially - Moles of carbon dioxide used
= 4.80 mol - (7.17 mol / 2) = 1.23 mol
We can determine the mass of excess carbon dioxide using the molar mass of carbon dioxide (44.01 g/mol):
Excess carbon dioxide = 1.23 mol * 44.01 g/mol = 54.1 kg
Therefore, the mass of the excess carbon dioxide left is approximately 54.1 kg.
Learn more about Limiting reactant, here:
https://brainly.com/question/10090573
#SPJ4
iven the biaryl product, select the two reactants that would give this product via a suzuki coupling. the unknown compound reacts with palladium tetrakis triphenyl phosphine, heat and sodium carbonate to give an alkene where each carbon is bonded to a benzene ring and a hydrogen and the stereochemistry is cis. select two reactants. an alkyne where carbon 1 is bonded to a benzene ring and carbon 2 is bonded to hydrogen. an alkene where carbon 1 is bonded to bromine and carbon 2 is bonded to benzene. the stereochemistry is cis. an alkene where carbon 1 is bonded to bromine and carbon 2 is bonded to benzene. the stereochemistry is trans. phenyl grignard tri butyl tin is bonded to benzene boronic acid is bonded to benzene an alkene is bonded to three hydrogens and one benzene ring.
Answers
Based on the given information, the two reactants that would give the described product via a Suzuki coupling are:
An alkyne where carbon 1 is bonded to a benzene ring and carbon 2 is bonded to hydrogen.
An alkene where carbon 1 is bonded to bromine and carbon 2 is bonded to benzene. The stereochemistry is cis.
The Suzuki coupling is a widely used cross-coupling reaction that involves the palladium-catalyzed cross-coupling of an organoboron compound (such as a boronic acid) with an organic halide or pseudo-halide. In this case, the reactants that can undergo the Suzuki coupling are the alkyne and the cis-alkene described above.
The alkene and alkyne provide the necessary carbon-carbon bond formation, while the benzene rings and hydrogens ensure the desired connectivity in the product. The palladium tetrakis triphenyl phosphine catalyst, heat, and sodium carbonate facilitate the coupling reaction, resulting in the formation of the biaryl product with the specified stereochemistry.
To know more about Suzuki coupling :
brainly.com/question/29857055
#SPJ11
What is the rate of production of product X if its concentration increases from 3.40x10-3 mol/L to 5.70x10-3 mol/L in 228.9 seconds.
Answers
The rate of production of product X is approximately 9.13 x \(10^{-6}\) mol/(L·s), indicating an increase in concentration by that amount per second.
To determine the rate of production of product X, we need to calculate the change in concentration of X divided by the change in time. The change in concentration is obtained by subtracting the initial concentration (3.40 x \(10^{-3}\) mol/L) from the final concentration (5.70x\(10^{-3}\)mol/L), resulting in a concentration change of 2.30 x \(10^{-3}\) mol/L.
Next, we divide the concentration change by the change in time, which is given as 228.9 seconds. Dividing the concentration change of 2.30 x \(10^{-3}\) mol/L by the time of 228.9 seconds gives us the rate of production of product X.
The rate of production of X is calculated as (5.70x\(10^{-3}\) mol/L - 3.40x10-3 mol/L) / 228.9 seconds, which simplifies to approximately 9.13x\(10^{-6}\)mol/(L·s). This indicates that for every second, the concentration of product X increases by 9.13x10-6 mol/L.
You can learn more about rate of production at
https://brainly.com/question/29886282
#SPJ11
is oatmeal a compound
Answers
Answer:
no
Explanation:
its made of a living organisim which in this case is oats
An experiment was conducted to estimate the effect of smoking on the blood pressure of a group of 37 cigarette smokers. The difference for each participant was obtained by taking the difference in the blood pressure readings at the beginning of the experiment and again five years later. The sample mean increase, measured in millimetres of mercury, was x = 9.1. The sample standard deviation was s = 5.5. Estimate the mean increase in blood pressure that one would expect for cigarette smokers over the time span indicated by the experiment. Find the 95% margin of error. (Round your answer to two decimal places
Answers
The 95% margin of error for the mean increase in blood pressure that one would expect for cigarette smokers over the time span indicated by the experiment is ±1.98 (rounded off to two decimal places).
The mean increase in blood pressure that one would expect for cigarette smokers over the time span indicated by the experiment can be estimated by using the formula;μ = x ± z(\(a^{2}\)) * σ/√n
Where;μ is the population mean increase.x is the sample mean increase.z(\(a^{2}\)) is the z-scoreα is the level of significanceσ is the population standard deviationn is the sample size.
Substituting the given values into the formula;μ = 9.1 ± 1.96 * 5.5/√37= 9.1 ± 1.98
The mean increase in blood pressure that one would expect for cigarette smokers over the time span indicated by the experiment lies between 7.12 to 11.08.
Hence, the estimated mean increase is between 7.12 to 11.08 millimeters of mercury.
The 95% margin of error can be calculated using the formula;
Margin of error (E) = z(\(a^{2}\)) * σ/√n
Margin of error (E) = 1.96 * 5.5/√37
Margin of error (E) = 1.98 (approximated to two decimal places).
To know more about standard deviation visit :
https://brainly.com/question/31850069
#SPJ11
What would be the temperature change if 3.0 g of water absorbed 15 J of heat? Specific Heat capacity of water= 4.18 J/gºC
Question 19 options:
a
None of the above
b
2.4 ºC
c
1.2 ºC
d
15 ºC
Answers
Exprese la concentración de una solución de H3PO4 al 30 % en masa y con una densidad de 1.39 g/mL en: M, y N.
Answers
Respuesta:
4.26 M; 12.8 N
Explicación:
Primer paso: Calcular la concentración volumétrica (Cv)
Usaremos la siguiente expression.
Cv = Cg × ρ
Cv = 30 g%g × 1.39 g/mL = 41.7 g%mL
Segundo paso: Calcular la molaridad
La concentración volumetrica es 41.7 g%mL, es decir, hay 41.7 gramos de soluto cada 100 mL de solución. Usaremos la siguiente fórmula para molaridad.
M = masa de soluto / masa molar de soluto × litros de solución
M = 41.7 g / 97.99 g/mol × 0.1 L = 4.26 M
Tercer paso: Calcular la normalidad
Usaremos la siguiente fórmula.
N = M × Z
donde Z para un ácido es igual al número de protones.
N = M × Z
N = 4.26 mol/L × 3 eq/mol = 12.8 N
