what are the structural characteristics of glycophorin that help it to maintain asymmetric orientation
Answers
The structural characteristics of glycophorin that help it to maintain asymmetric orientation is the center of the proteins contains hydrophobic amino acid residue.
The glycophorin A is the type I single membrane protein. the glycophorin plays an important tole in the process of the invasion of the red blood cells by the malaria. glycophorin is the membrane of the red blood cell. the membrane of the spanning protein. glycoprotein of plasma is situated with the sugar residue.
Thus , in the glycophorin a segment in the contains protein residue of 75 to 93. theses are hydrophobic residue of amino acid. it is a transmembrane segment.
To learn more about glycophorin here
https://brainly.com/question/29588507
#SPJ4
Related Questions
Please! I need help
Describe how water molecules can hydrate various substances.
Answers
Answer:
The two hydrogen atoms and one oxygen atom within water molecules (H2O) form polar covalent bonds. ... As a result of water's polarity, each water molecule attracts other water molecules because of the opposite charges between them, forming hydrogen bonds.
Explanation:
Not my work, but I hope this helps!
The density of water is ____________.
Answers
Answer:
The density of water is 1 g/mL.
Explanation:
M(s) + 3Ag+(aq) →3Ag(s) + M3+(aq) E° = +2.46 V
Ag+(aq) + e-→Ag(s) E° = +0.80 V
According to the information above, what is the standard reduction potential for the half-reaction M3+(aq) + 3e-→M(s)?
-1.66 V
-0.06 V
0.06 V
1.66 V
Answers
The standard reduction potential for the half-reaction M3+(aq) + 3e- → M(s) can be determined by subtracting the standard reduction potential of the half-reaction Ag+(aq) + e- → Ag(s) from the overall reaction's standard potential.
Given:
E°(overall reaction) = +2.46 V (from the balanced equation)
E°(Ag+(aq) + e- → Ag(s)) = +0.80 V (given)
To find E°(M3+(aq) + 3e- → M(s)):
E°(M3+(aq) + 3e- → M(s)) = E°(overall reaction) - E°(Ag+(aq) + e- → Ag(s))
E°(M3+(aq) + 3e- → M(s)) = +2.46 V - (+0.80 V)
E°(M3+(aq) + 3e- → M(s)) = +1.66 V
Therefore, the standard reduction potential for the half-reaction M3+(aq) + 3e- → M(s) is +1.66 V.
Learn more about standard reduction potential here : brainly.com/question/31868529
#SPJ11
How does examining past data help prepare people for feature weather hazards?
Answers
Answer:
It lets people prepare for future weather hazards:
- If, in certain months and on certain dates, the past data shows that there's a history of rain or heat stroke on those days, people can prepare in the future for those events.
- They can also expect wind speeds, temperatures and stuff like that!
helpp please it's just 5 small questions.

Answers
Dissolved salt and water can be separated by the method 2 but not method 1. Blue red and yellow contain only one dye.
What is chromatography?Chromatography is a technique used to separate components of a mixture based on their different interactions with a stationary phase and a mobile phase. The stationary phase is a material that is fixed in place and the mobile phase is a liquid or gas that passes over the stationary phase. The components of the mixture interact differently with the stationary phase and mobile phase, causing them to move at different rates and become separated from each other.
Method 1;
Sand and water
Sand an iron fillings
Sugar and salt
Method 2;
Sugar and salt both dissolved in water
Learn more about distillation:brainly.com/question/29037176
#SPJ1
PLS ANSWER THANK YOUU

Answers
Answer:
Chicken Noodle Soup
Explanation:
The rest do have other things in them but are dissolved unlike the chicken noodle soup.
Hope this Helps!!
:D
4. At what temperature will 5.00 g of Cl, exert a pressure of 900. torr at a volume of 750 ml?
Answers
Answer:
p=27.8atm
Explanation:
P = 27.8atm
At what temperature will 5.00 g of Cl2 exert a pressure of 900. mmHg at a volume of 750.
How does a nuclear power plant create electricity?*
A: A nuclear reaction burns fuel
B: A nuclear reaction caused water to flow, which is then what creates electricity
C: A nuclear reaction creates heat which is applied to water to create steam
D: A nuclear reaction creates an electrical current
Answers
Answer:
CExplanation:
Answer:
hi jajsbbsjsmsksjsksksjsjdkkdkdndndnxjsjsksksksjsksmsnznnsnsnznsjsjshsjjajannananabababababajajajajajabananabbananajanabajajakmaka
Which are characteristics of a prokaryotic cell? Select three options. contains DNA lacks DNA contains ribosomes lacks ribosomes contains a nucleus lacks a nucleus
Answers
Answer:
Prokaryotic cells contain DNA, contain ribosomes, and lack a nucleus.
Answer:
Contains DNA
Contains Ribosomes
lacks a nucleus
Explanation: I took the quiz on edge
f the barometer read 765.2 mmhg when the measurement in in the figure below took place, what is the pressure of the gas in the flask in kilopascals?
Answers
The pressure of the gas in the flask in kilopascals is given by the term 100.3 kPa, option E.
The pressure of any gas is a crucial characteristic. In contrast to qualities like viscosity and compressibility, we have some experience with gas pressure. Every day, the TV meteorologist reports the value of the atmosphere's barometric pressure.
We have included numerous slides on gas pressure in the Beginner's Guide since comprehending what pressure is and how it works is so essential to understanding aerodynamics. It is possible to investigate how static air pressure varies with altitude using an interactive atmosphere simulator. You can see how the pressure changes around a lifting wing using the FoilSim software.
height difference, h, indicates pressure of gas relative to atmospheric pressure.
h= 13mm
barometric pressure =765.2mmHg (atmosphere)
-from the picture, we can see that atmospheric pressure is greater than the gas pressure. so we minus
765.2mm - 13mm= 752.2mmHg
752.2mmHg * (101.3kPa / 760mmHg) = 100.3kPa.
Learn more about Pressure of gas:
https://brainly.com/question/30003139
#SPJ4
Complete question:
If the barometer read 765.2 mmHg when the measurement in in the Figure below took place, what is the pressure of the gas in the flask in kilopascals?
A. 7.55 kPa
B. 102.4 kPa
C. 1.007 kPa
D. 752.2 kPa
E. 100.3 kPa
what is 23.54 kg x 9.8 m/s2
Answers
Answer:
230.692
Explanation:
23.54kg*9.8m/s²= 230.692
Answer:
F = 230.69 N
Explanation:
Given data:
Mass = 23.54 Kg
Acceleration = 9.8 m/s²
Force = ?
Solution:
Mass ×Acceleration is equal to force.
F = 23.54 Kg × 9.8 m/s²
F = 230.69 Kg.m/s²
Kg.m/s² = N
F = 230.69 N
hi pls I need an answer my final exams is tomorrow pls help me balance the equation Cu + H₂SO4 → CuSO4 +S0₂ + H₂
Answers
Answer:
Cu + H₂SO4 → CuSO4 + SO2 + H₂
To balance this equation, we need to ensure that the number of atoms of each element is equal on both sides of the equation. Here's how to balance it step by step:
Balance the Copper atoms: There is 1 Copper atom on each side, so no changes are needed.
Cu + H₂SO4 → CuSO4 + SO2 + H₂
Balance the Sulfur atoms: There is 1 Sulfur atom on each side, so no changes are needed.
Cu + H₂SO4 → CuSO4 + SO2 + H₂
Balance the Hydrogen atoms: There are 2 Hydrogen atoms on the right side, but only 1 on the left. We can balance this by adding a coefficient of 2 in front of H₂.
Cu + H₂SO4 → CuSO4 + SO2 + 2H₂
Balance the Oxygen atoms: There are 4 Oxygen atoms on the right side, but only 2 on the left. We can balance this by adding a coefficient of 2 in front of CuSO4 and a coefficient of 1/2 in front of SO2.
Cu + H₂SO4 → 2CuSO4 + SO2 + 2H₂
Now the equation is balanced, with 1 Copper atom, 1 Sulfur atom, 4 Hydrogen atoms, and 6 Oxygen atoms on each side. I hope this helps, and good luck on your final exam tomorrow!
Answered By Unish ©
Verified Answer ✅
Please Mark As Brainliests
It’s due tomorrow and I don’t know how to do it.

Answers
question 1 a spreadsheet cell contains the coldest temperature ever recorded in new zealand: -22 °celsius. what function will display that temperature in fahrenheit?
Answers
When the temperature conversion function =CONVERT(-22, "C", "F") is applied, a reading of -22 °C in Fahrenheit is displayed. On a variety of scales, including the Fahrenheit and Celsius systems, temperature is a unit that is used to denote hotness or coolness.
Heat energy will logically go from a hotter (body with a higher temperature) to a colder (body with a lower temperature) according to temperature (one at a lower temperature).
A temperature is a measurement used to express how hot or cold something is. It demonstrates how heat energy naturally flows from a hotter body to a cooler body and can be expressed in terms of any number of arbitrary scales (one at a lower temperature).
A match is burning at a far greater temperature than an iceberg, yet an iceberg has a significantly higher total heat energy than a match. Temperature is not the same as the energy of a thermodynamic system.
The temperature, along with pressure, density, and other similar properties, is referred to as an intense property as opposed to extensive characteristics like mass or volume—one that is independent of the quantity of stuff being addressed.
To know more about temperature:
brainly.com/question/23411503
#SPJ4
PLEASE HELP!
Perform the following operation
and express the answer in
scientific notation.
5.450x10-4 x 3.550x 10-7
[ ? ]x10[?]

Answers
Answer:
\(5.450 \times {10}^{ - 4 } \times 3.550 \times {10}^{ - 7} \\ 19.3475 \times {10 }^{ - 11} \\ 1.935 \times {10}^{?10} \)
write 5.5 x 104 in standard form
Answers
Answer: 55,000
Explanation:
Scientific Notation: 5.5 x 10⁴
Standard form: move the decimal 4 places to the right
5 5 0 0 0 0.
= 55,000
Answer:
5.72×10^2
Explanation:
5.5×104
=55×104
=5720÷10
=572
=5.72×10^2
2 moles of NO, was placed in an empty I dm' bottle and allowed to reach equilibrium according to the equation:
At equilibrium, 1.2 moles of N,O, dissociated. Calculate the value of the equilibrium constant for the reaction at that
temperature.
Answers
2NO(g) ⇌ N2(g) + O2(g)
According to the problem statement, 2 moles of NO were placed in a 1 dm^3 bottle and allowed to reach equilibrium, and at equilibrium, 1.2 moles of NO had dissociated. This means that the initial concentration of NO was:
[NO]initial = 2 mol / 1 dm^3 = 2 M
And the concentration of NO at equilibrium is:
[NO]equilibrium = (2 - 1.2) mol / 1 dm^3 = 0.8 M
Since the stoichiometry of the balanced equation is 2:1:1 for NO, N2, and O2, respectively, the equilibrium concentrations of N2 and O2 will also be 0.6 M.
The equilibrium constant (Kc) can be calculated using the equilibrium concentrations of the reactants and products, raised to the power of their stoichiometric coefficients. Therefore:
Kc = ([N2][O2]) / ([NO]^2)
Substituting the equilibrium concentrations into the equation, we get:
Kc = (0.6 M x 0.6 M) / (0.8 M x 0.8 M)
Kc = 0.5625
Therefore, the value of the equilibrium constant for the reaction at that temperature is 0.5625. Note that the units of Kc depend on the stoichiometry of the balanced equation. Since the stoichiometric coefficients are all 1, the units of Kc in this case are M^-1
For the equation below, write the oxidation half-reaction and the reduction half-reaction. 2Na + Cl2 -----> 2NaCl
Answers
Answer:
Oxidation half-reaction : Na(s) → Na⁺ + 1 e-
Reduction half-reaction: Cl₂ + 2 e- → 2 Cl⁻
Explanation:
Oxidation half-reaction: solid sodium (Na(s)) has an oxidation number of 0. It loses 1 electron and forms the cation Na⁺. So, the half-reaction is the following:
Na(s) → Na⁺ + 1 e-
Reduction half-reaction: chlorine gas (Cl₂) has an oxidation number of 0. Each atom of Cl gains 1 electron to form two Cl⁻ ions, according to the following half-reaction:
Cl₂ + 2 e- → 2 Cl⁻
The total oxidation-reduction reaction is obtained by adding the oxidation half-reaction multiplied by 2 (to balance the electrons) and the reduction half-reaction, as follows:
2 x (Na(s) → Na⁺ + 1 e-)
Cl₂ + 2 e- → 2 Cl⁻
--------------------------------
2Na(s) + Cl₂ → 2NaCl
Alex's teacher showed him a model of gas particles in a sealed container. How should Alex change the model to show the particles of this gas at a higher temperature?
Answers
Answer:
Increased volume of particles in the containergreater vibration of particlesExplanation:
At higher temperature, the particles of the gas would be more active and vibrate more, or even have greater collisions. Alex can indicate this in the altered model to depict higher temperature.
Consequently, Charles law gives meaning to why there would be an increased volume of gas in the stable pressurized container, if the temperature were to be increased.
I hope this explanation was clear and concise?
which two changes would make this reaction product-favored
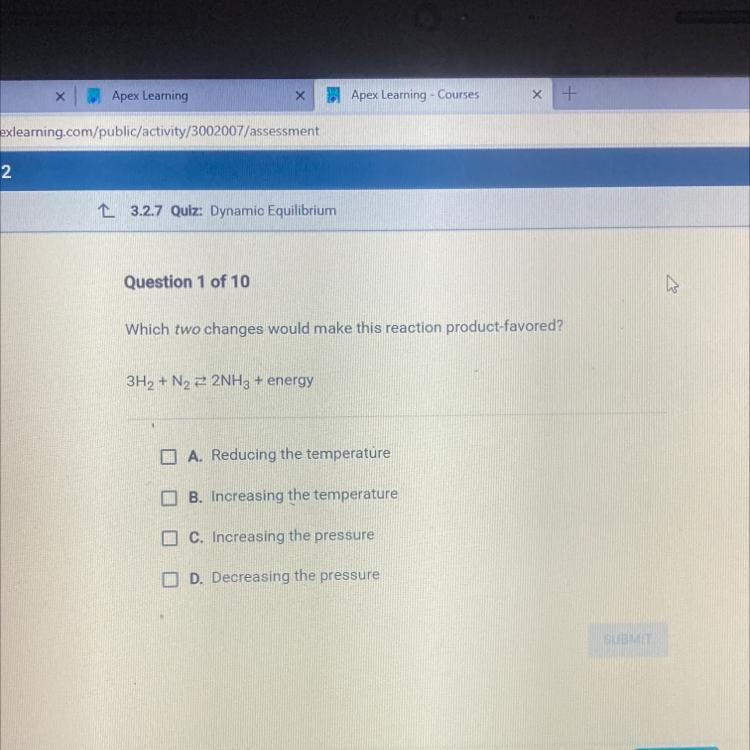
Answers
The changes that are product favored are;
Reducing the temperature
Increasing the pressure
Dynamic equilibrium in chemical reactionA chemical reaction is said to be in a state of dynamic equilibrium when the forward and reverse reactions are occurring at the same rate and there is no net change over time in the reactant and product concentrations. It is a dynamic state because individual molecules are constantly conducting reactions, despite the fact that the concentrations of reactants and products are constant.
In a chemical reaction, reactants undergo a forward reaction in which they are transformed into products, and products undergo a reverse reaction in which they are transformed back into reactants.
Learn more about dynamic equilibrium:https://brainly.com/question/1298350
#SPJ1
Why do you have to have a certain number of reactants on one side?
Answers
Answer:Because you need that certain number and double the number of bread to get the reactants off of the sides
Explanation: 4 bread reactants- 2meat reactants- 2 cheese reactants= 2 products
The reason for the presence of a certain number of reactants on one side is to ensure that the chemical equation is balanced.
In a balanced chemical equation, the number of atoms of each element on the reactant side must be equal to the number of atoms of that element on the product side.
Consider the equation:
\(2H_2 + O_2 - > 2H_2O.\)
This equation represents the reaction of hydrogen gas (\(H_2\)) with oxygen gas (\(O_2\)) to form water (\(H_2O\)).
To balance this equation, double the coefficient in front of \(H_2O\) on the product side, resulting in 4 hydrogen atoms and 2 oxygen atoms on both sides.
Therefore, having a certain number of reactants on one side is necessary to ensure that a chemical equation is balanced, satisfying the law of conservation of mass.
To know more about the balanced chemical equation, visit:
https://brainly.com/question/29130807
#SPJ3
Find an example of an ester used as a fragrance or flavoring and give the name, condensed structural formula, and flavor of your chosen ester.
Answers
The example of an ester used as a fragrance is isopentyl acetate and it is present in banana. Details about esters can be found below.
What are esters?Esters are compounds most often formed by the condensation of an alcohol and an acid and has the functional group carbon-oxygen double bond joined via carbon to another oxygen atom.
Esters are naturally found in certain fruits and can be used as major constituent of perfumes. An example of ester used as fragrance is isopentyl acetate.
Isopentyl acetate has the molecular formula of C7H14O2 and has a banana flavor.
Learn more about esters at: https://brainly.com/question/10840252
#SPJ1
Balancing Chemical Equation
CuCl2+H2S=CuS+HCl
Answers
Answer:
CuCi2 + H2S ⇒ CuS + 2HCi
Explanation:
In order to balance a chemical equation you need to make sure to have the same number of atoms on each side by multiplying on both sides.
CuCi2 + H2S = CuS + HCi
Cu = 1
Ci = 2
H = 2
S = 1
Product
Cu = 1
S = 1
H = 1 × 2 = 2
Ci = 1 × 2 = 2
Since all elements have a balanced amount of atoms, the equation is now balanced.
CuCi2 + H2S ⇒ CuS + 2HCi
Hope this helps.
A chemical equation is said to be balanced if the quantity of each type of atom in the reaction is the same on both the reactant and product sides. In a balanced chemical equation, the mass and the charge are both equal. Here the balanced equation is CuCl₂ + H₂S → CuS + 2HCl.
A balanced chemical equation, in which the masses of the reactants and products are equal, contains the same amount of atoms of each element on both sides of the equation. In other words, both sides of the reaction have an equal balance of mass and charge.
The chemical equation is said to be balanced if there are no inequalities. Here the balanced equation is:
CuCl₂ + H₂S → CuS + 2HCl
To know more about balanced equation, visit;
https://brainly.com/question/14812568
#SPJ6
Weathering and Erosion Unit Test
Explain how crustal deformation builds landforms.
O
When two tectonic plates start to push into each other they can rise up and build mountains, or sink under
and create deep valleys.
O When wind and rain slowly wear away rock, they leave behind new looking landforms.
If
o
When two tectonic plates slide away from each other the inner liquid layer of the mantle comes to the
surface as lava and creates new islands.
It
When sediments are laid down over vast areas the forces involved create rock over a very long period of
time.
It
HELP ME ASAPPP PLEASEEE
Answers
Crustal deformation builds landforms when two tectonic plates start to push into each other they can rise up and build mountains, or when they sink under, they create valleys.
How crustal deformation builds landforms?Tectonic pressure in a crust can cause folding. Folding can end up with the formation of valleys and mountains so we can conclude that when two tectonic plates start to push into each other they can rise up and build mountains, or when they sink under, they create deep valleys.
Learn more about plates here: https://brainly.com/question/16939139
Two scientists were interested in discovering the effect of caffeine on heart rate. Identify the following:
Independent Variable :
Dependent Variable :
Constant:
Control:
Answers
Discovering the effect of caffeine on heart rate. The Independent Variable is caffeine, dependent Variable is heart beat, Control is group given no caffeine and constant is same amount of caffeine.
What is Independent variable?Independent variable is defined as varible which is changed by the experimenter.
What is Dependent Variable?Dependent variable is defined as those variable which depends on the independent variable.
What is Control group?It is defined as that group which do not receive experimental treatment.
What is Constant Variable?It is that variable which is kept unchanged for all types of groups in the experiment.
Thus, we concluded that the the Independent Variable is caffeine, dependent Variable is heart beat, Control is group given no caffeine and constant is same amount of caffeine.
learn more about Variables:
https://brainly.com/question/1479694
#SPJ9
1. Using PbS + 3PbO -> 3Pb + SO2a. How many moles of Pb will form from 17 moles of PbO (excess PbS)?b. How many moles of PbO are needed to make 27.4 moles of SO2 (excess PbS)c. Give 17.6 moles of PbS and 36 moles of PbO, which is the limiting reactant (show mathematical proof). It’s
Answers
Answer:
a. 25.5 moles of Pb will be formed.
b. 54.8 moles of PbO are needed.
c. PbS is the limiting reactant.
Explanation:
1st) It is necessary to write the balanced chemical reaction:
\(PbS+2PbO\rightarrow3Pb+SO_2\)From the balanced chemical reaction we know that 1 mole of PbS reacts with 2 moles of PbO to produce three moles of Pb and 1 mole of SO2.
2nd) Using the stoichiometry of the reaction and a mathematical rule of three, we can calculate the moles of Pb that will be produced from 17 moles of PbO and excess of PbS:
\(\begin{gathered} 2molesPbO-3molesPb \\ 17molesPbO-x=\frac{17molesPbO*3molesPb}{2molesPbO} \\ x=25.5molesPb \end{gathered}\)So, 25.5 moles of Pb will be formed.
3rd) Using the stoichiometry of the reaction and a mathematical rule of three, we can calculate the moles of PbO that are needed to make 27.4 moles of SO2 in excess of PbS:
\(\begin{gathered} 1molSO_2-2molPbO \\ 27.4molSO_2-x=\frac{27.4molSO_2*2molPbO}{1molSO_2} \\ x=54.8molPbO \end{gathered}\)So, 54.8 moles of PbO are needed.
4th) Using the stoichiometry of the reaction we can calculate the limiting reactant:
- Calculation from 17.6 moles of PbS:
\(\begin{gathered} 1molPbS-2molPbO \\ 17.6molPbS-x=\frac{17.6molPbS*2molPbO}{1molPbS} \\ x=35.2molPbO \end{gathered}\)From the stoichiometry of the reaction 1 mol of PbS reacts with 2 moles of PbO, so the 17.6 moles of PbS will need 35.2 moles of PbO to react properly, but we have 36g of PbO, so PbO will be the excess reactant and PbS the limiting reactant.
- Calculation from 36 moles of PbO:
We can do this calculation to confirm the previous one:
\(\begin{gathered} 2molPbO-1molPbS \\ 36molPbO-x=\frac{36molPbO*1molPbS}{2molPbO} \\ x=18molPbS \end{gathered}\)In this case, we can see that 36 moles of PbO will need 18 moles of PbS to react properly, but we only have 17.6 moles of PbS. Here we confirm that PbS is the limiting reactant.
The atomic number of polassium and
calcium is 19 and 20 respectively and they
belong to the same period.
Who among them would have smaller atomic size
Answers
Explanation:
Atomic radius decreases from left to right in a period.
Therefore Calcium would have a smaller atomic size.
What happens when thermal energy is removed from a substance?
Answers
Answer:
removing thermal energy will cause its particles to move more slowly and its temperature to drop
When thermal energy is removed from a substance the energy of the system will be low and particles of the substance will not move properly because of low energy.
What is thermal energy?Thermal energy is a type of energy that is mainly produced by the generation of the temperature as it is only responsible for the production of heat which is responsible for thermal energy production.
Energy provides the particles of a substance to move from one place to another place which is not possible without energy or heat or thermal energy and the process will not be complete.
Therefore, the energy of the system will be low and particles of the substance will not move properly because of the low energy When thermal energy is removed from a substance.
Learn more about thermal energy, here:
https://brainly.com/question/11278589
#SPJ2
In the reaction inside Flask 3, you observed that this was the neutralization reaction: HNO3 + NaOH -> NaNO3 + H2O What is the conjugate base in this reaction? H2O HNO3 NaOH NaNO3
Answers
A neutralization reaction is a chemical reaction in which an acid and a base react with each other to form a salt and water. The reaction is typically exothermic and pH of the resulting solution will be neutral/7.
In the neutralization reaction given:
HNO3 + NaOH -> NaNO3 + H2O
To identify the conjugate base, let's first look at the acid and base in the reaction. HNO3 (nitric acid) is the acid, and NaOH (sodium hydroxide) is the base.
When an acid loses a proton (H+), it forms its conjugate base. In this reaction, HNO3 loses a proton and forms the conjugate base NO3- (nitrate ion). The product that contains this conjugate base is NaNO3 (sodium nitrate).
So, the conjugate base in this reaction is found in NaNO3.
To know more about conjugate base, visit:
https://brainly.com/question/30086613
#SPJ11
which of these is an example of a chemical change?
Answers
Answer:
No answer choices but Chemical changes are changes in which can't be undone an example would be a rotting food(won't be fresh ever again) or burning paper(ashes are ashes not paper)
Explanation:
Hope this helps :D