What are the number of protons, electrons, and neutrons in one atom of neon?
protons
electrons
neutrons
Answers
Answer:
protons- 10
electrons- 10
neutrons- 10
Explanation:
no of protons/ electrons = atomic number
no of neutrons = atomic mass - atomic number
Related Questions
3. Could a molecule
contain only one atom?
Answers
simplify the expression by combining like terms: 5b2 + 9b + 10 + 3b + 2b2−4.
Answers
Answer: 7b² + 12b + 6
Explanation:
I am going to assume that by 5b2 and 2b2, it is meant to be 5b² and 2b².
Given:
5b² + 9b + 10 + 3b + 2b² − 4
Reorder by like terms (terms that have the same degree):
5b² + 2b² + 9b + 3b + 10 − 4
Combine like terms (add and/or subtract terms with the same degree):
➜ 5 + 2 = 7
➜ 9 + 3 = 12
➜ 10 - 4 = 6
7b² + 12b + 6
To simplify the expression by combining like terms, we need to group together the terms Catalysis that have the same variable and the same exponent. 5b2 + 9b + 10 + 3b + 2b2 − 4 the results from step 2: 7b² + 12b + 6.
The expression given has terms with different variables and exponents. To simplify the expression, we need to group together the terms that have the same variable and exponent. So, we rearrange the terms in the expression by collecting the like terms. In this case, we group the b2 terms together and the b terms together. We also group the constant terms together.
Identify like terms. In this case, the like terms are the terms with the same variable and exponent. We have three sets of like terms: b² terms (5b² and 2b²), b terms (9b and 3b), and constants (10 and -4).
Combine the like terms by adding or subtracting them. - Add the b² terms: 5b² + 2b² = 7b - Add the b terms: 9b + 3b = 12b- Add the constants: 10 + (-4) = 6
To know more about Catalysis visit:
https://brainly.com/question/30417381
#SPJ11
what physical properties can be used to separate heterogeneous mixture's?
Answers
Hope it help you, have a nice day
I need this answer somebody Please help me
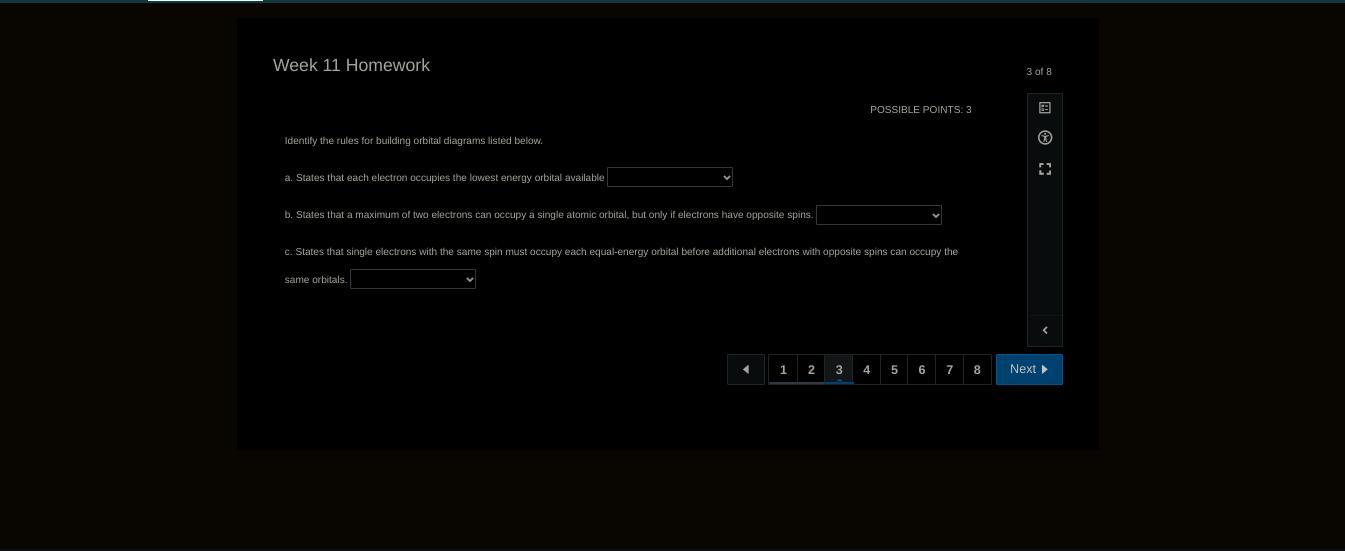
Answers
The correct responses that describe the various rules in the filling of orbitals are;
1) Aufbau principle
2) Pauli exclusion principle
3) Hund's rule.
What is the orbital?The orbital has to do with the region in space where there is a high probability of finding the electron. We have to know that that looking at the orbitals, we can know how electrons are arranged in the atoms in order of increasing energy.
The Aufbau principle shows us that when we try to fill the electrons into orbitals, we must start from the orbital that has the lowest energy. The Pauli exclusion principle states that no two electrons in an atom can have the same value for all the four quantum numbers. In other words, electrons in the same orbital must have anti parallel spins.
Also, when we are filling electrons into orbitals that have the same energy, we fill them in singly first. This is the statement of the Hund's rule.
Learn more about orbitals:https://brainly.com/question/18914648
#SPJ1
mass of 2 into 10 to power 21 number of atoms of an element is 0.4 gram what is the mass of 0.5 mole of the elements
Answers
The mass of 0.5 mole of the element is approximately 6.025 grams.
To calculate the mass of 0.5 mole of the element, we need to know the molar mass of the element.
Given that the mass of 2 x 10^21 atoms of the element is 0.4 grams, we can use this information to find the molar mass.
The number of atoms in 1 mole of any substance is given by Avogadro's number, which is approximately 6.022 x 10^23 atoms/mol.
First, we calculate the molar mass of the element using the given information:
Molar mass = Mass of 2 x 10^21 atoms / Number of moles of 2 x 10^21 atoms
Molar mass = 0.4 g / (2 x 10^21 atoms / (6.022 x 10^23 atoms/mol))
Molar mass ≈ 0.4 g / (3.32 x 10^-2 mol)
Molar mass ≈ 12.05 g/mol
Now that we know the molar mass of the element is approximately 12.05 g/mol, we can calculate the mass of 0.5 mole of the element:
Mass = Molar mass x Number of moles
Mass = 12.05 g/mol x 0.5 mol
Mass = 6.025 grams
for more such questions on element
https://brainly.com/question/28376204
#SPJ8
a.) [Ar]4s13d104p25p1
Express your answer as a chemical symbol.
b.) [Kr]5s24d25p1
Express your answer as a chemical symbol.
Answers
The name and chemical symbol of the given element whose electronic configurations are shown is:
a. Germanium and its symbol is Ge.
b. Niobium and its symbol is Nb.
What is the chemical symbol of an element?The chemical symbol of an element is the symbol that is used to represent the atom of the element usually based on the name o the element.
The name and chemical symbol of the given element whose electronic configurations are shown is determined as follows:
a.) [Ar]4s¹3d¹⁰4p²5p¹
The atomic number of the element is 32
The element whose atomic number is 32 is Germanium and its symbol is Ge.
b.) [Kr]5s²4d²5p¹
The atomic number of the element is 41
The element whose atomic number is 32 is Niobium and its symbol is Nb.
Learn more about chemical symbol at: https://brainly.com/question/28376204
#SPJ1
Which of the following is an oxidation-reduction reaction? Cu(s) + 2AgNO_3(aq) rightarrow 2Ag (s) + Cu(NO_3)_2(aq) H_2CO_3(aq) + Ca(NO_3)_2(aq) rightarrow 2HNO_3(aq) + CaCO_3(s) Ag NO_3 (aq) + HCl (aq) rightarrow AgCl (s) + HNO_3 (aq) Ba(C_2H_3O_2) 2(aq) + Na_2SO_4 (aq) rightarrow BaSO_4(s) + 2NaC_2H_3O_2(aq) HCl(aq) + NaOH (aq) rightarrow H_2O (I) + NaCl (aq)
Answers
Cu (s) + 2AgNO₃ (aq) → 2Ag (s) + Cu(NO₃)₂ (aq) is an oxidation -reduction reaction
An oxidation-reduction reaction is a chemical reaction that involves a transfer of electrons between two atoms or molecules. It is any chemical reaction in which the oxidation number of a molecule, atom, or ion changes by gaining or losing an electron. For a reaction to be an oxidation-reduction reaction, the oxidation state of an individual atom should be 0.
In H₂CO₃ (aq) + Ca(NO₃)₂ (aq) → 2HNO₃ (aq) + CaCO₃ (s)
Carbon on the left has an oxidation state of -4, on the right it has also oxidation state -4
Nitrogen has on the left an oxidation state of +5, on the right it has also an oxidation state of +5
Calcium has on the left an oxidation state of +2, on the right also an oxidation state of +2
⇒ There is no change of oxidation number so this is NOT a redox reaction
In AgNO₃(aq) + HCl (aq) → AgCl (s) + HNO₃
Silver (Ag) on the left has an oxidation state of +1, on the right it has also oxidation state +1
Nitrogen has on the left an oxidation state of +5, on the right it has also an oxidation state of +5
Cl has on the left an oxidation state of -1, on the right it has also an oxidation state of -1
⇒ There is no change of oxidation number so this is NOT a redox reaction
Ba(C₂H₃O₂)₂ (aq) + Na₂SO₄(aq) → BaSO₄ (s) + 2NaC₂H₃O₂(aq)
Na has on the left an oxidation state of +1, on the right it has also an oxidation state of +1
Ba has on the left an oxidation state of +2, on the right it has also an oxidation state of +2
S has on the left an oxidation state of +6, on the right it has also an oxidation state of +6
⇒ There is no change of oxidation number so this is NOT a redox reaction
Cu (s) + 2AgNO₃(aq) → 2Ag (s) + Cu(NO₃)₂ (aq)
Cu has on the left an oxidation state of 0, on the right it has an oxidation state of +2
Ag has on the left an oxidation state of +1, on the right it has also an oxidation state of 0
⇒ There is a change of oxidation number so this is a redox reaction
Oxidizing agent: Ag in AgNO₃ ; Reducing agent: Cu
HCl (aq) + NaOH (aq) → H₂O (l) + NaCl (aq)
Na has on the left an oxidation state of +1, on the right it has also an oxidation state of +1
Cl has on the left an oxidation state of -1, on the right it has also an oxidation state of -1
To know more about oxidation-reduction reaction here
https://brainly.com/question/29771993
#SPJ4
N2O4 ⇌ 2NO2
for the following reaction at 373 K, Kc = 0.36. If initial concentration of N2O4 is 0.1 mol dm^-3, what is the equilibrium concentration of NO2? (Is there a way to solve this without using quadratics?)
Answers
Okay, let's solve this step-by-step without using quadratics:
1) The equilibrium constant Kc = 0.36 means the equilibrium lies to the left. So there will be more N2O4 than NO2 at equilibrium.
2) The initial concentration of N2O4 is 0.1 mol dm^-3. Let's call this [N2O4]initial.
3) At equilibrium, the concentrations of N2O4 and NO2 will be [N2O4]equil and [NO2]equil respectively.
4) We know the equilibrium constant expression for this reaction is:
Kc = ([NO2]equil)^2 / [N2O4]equil
5) Setting this equal to 0.36 and plugging in 0.1 for [N2O4]initial, we get:
0.36 = ([NO2]equil)^2 / (0.1 - [NO2]equil)
6) Simplifying, we get:
0.036 = [NO2]equil^2
7) Taking the square root of both sides, we get:
[NO2]equil = 0.06 mol dm^-3
So the equilibrium concentration of NO2 is 0.06 mol dm^-3.
Let me know if you have any other questions! I can also provide a more step-by-step explanation if needed.
Which nitrogen base sequence is the partner of T-C-A-G-C-A?
• A-C-G-A-C-T
• C-A-G-A-T-G
• A-G-T-C-G-T
• T-C-A-G-C-A
Help pls
Answers
According to the complementary base pairing in DNA, the nitrogen base sequence which is the partner of T-C-A-G-C-A is A-G-T-C-G-T.
What is DNA?DNA is a hereditary material which is present in human beings as well as all other living organisms. Every cell which is present in an organism's body has DNA which is the same. Most of the DNA is situated in the cell's nucleus and small amount of it can be found in the cell's mitochondria as well.
Information which is stored in DNA is stored as codes made up of four chemical bases namely, adenine, thymine , cytosine and guanine.Human DNA consists of 3 billion bases .The order of the bases determines information which is required for building and maintaining an organism.
DNA bases are capable of pairing up with each other. Adenine pairs with thymine and guanine pairs up with cytosine .Each base is also attached to a sugar molecule and a phosphate group. A base, phosphate sugar are together called as nucleotides.
Learn more about DNA,here:
https://brainly.com/question/14315652
#SPJ9
What is the pH of a substance that has a hydrogen ion concentration of 9. 7x10-3 M?
Answers
Answer:
\( \huge{ \boxed{ \boxed{2.01}}}\)
Explanation:
pH is defined as the negative logarithm of the hydrogen ion concentration of a solution. That is,
\( \bold{pH = -log([{H}^{+}])} \)
From the question
\( [{H}^{+}] \) = 9.7 × 10-³ M
We have,
\(pH = - log(9.7 \times {10}^{ - 3} ) \\ = 2.01\)
We have the final answer as,
2.01what is the δh of the following hypothetical reaction? 2a(s) b2(g) → 2ab(g) given: a(s) b2(g) → ab2(g) δh = -179.9 kj 2ab(g) b2(g)
Answers
We can use Hess's Law to find the ΔH of the reaction. Hess's Law states that if a reaction can be expressed as the sum of a series of steps, then the ΔH for the overall reaction is the sum of the ΔH values for each step.
The given reaction can be broken down into two steps:
Step 1: a(s) + b2(g) → ab2(g) ΔH = -179.9 kJ/mol (Given)
Step 2: ab2(g) → 2ab(g) + b2(g) ΔH = ?
To obtain the overall reaction, we need to flip the direction of the second step and multiply its ΔH by -1:
2ab(g) + b2(g) → ab2(g) ΔH = -(-ΔH) = ΔH
Now, we can add the two steps together to get the overall reaction:
2a(s) + 2b2(g) → 2ab(g) ΔH = ΔH(step 1) + ΔH(step 2)
ΔH = -179.9 kJ/mol + ΔH(step 2)
Therefore, to find the ΔH of the overall reaction, we need to find the ΔH for Step 2.
From the chemical equation of Step 2, we see that one mole of ab2(g) is converted into two moles of ab(g) and one mole of b2(g), which means that the reaction requires the breaking of one mole of the AB bond in ab2(g) and the formation of two A-B bonds in ab(g), as well as the formation of one B-B bond in b2(g).
The overall bond breaking requires energy, while bond formation releases energy. The bond energy data for the relevant bonds can be used to calculate the enthalpy change of the reaction:
ΔH = 2*(bond energy of AB in ab(g)) + (bond energy of B-B in b2(g)) - (bond energy of AB in ab2(g))
Looking up the bond energies and substituting the values, we get:
ΔH = 2*(188 kJ/mol) + (193 kJ/mol) - (389 kJ/mol) = -200 kJ/mol
Therefore, the ΔH for the hypothetical reaction is -179.9 kJ/mol + (-200 kJ/mol) = -379.9 kJ/mol.
The negative sign indicates that the reaction is exothermic, releasing energy in the form of heat.
Learn more about reaction here:
https://brainly.com/question/14025220
#SPJ11
If the ksp for ca3(po4)2 is 8. 6×10−19, and the calcium ion concentration in solution is 0. 0023 m, what does the phosphate concentration need to be for a precipitate to occur?
Answers
The answer is the phosphate concentration of \(8.40\times10^{-6}M\)
Calcium phosphate \($\left(\mathrm{Ca}_{3}\left(\mathrm{PO}_{4}\right)_{2}\right)$\) is dissociate to form
calciumion \($\left(\mathrm{Ca}^{2+}\right)$\) and phosphate ion \($\left(\mathrm{PO}_{4}^{3-}\right)$\).
\($\mathrm{Ca}_{2}\left(\mathrm{PO}_{4}\right)_{2}(\mathrm{~s}) \longrightarrow 3 \mathrm{Ca}^{2+}(\mathrm{aq})+2 \mathrm{PO}_{4}^{3-}(\mathrm{aq})$\)
Soluability product expression of \($\mathrm{Ca}_{3}\left(\mathrm{PO}_{4}\right)_{2}$\) is,
\($K_{s p}=\left[\mathrm{Ca}^{2+}\right]^{3} \times\left[\mathrm{PO}_{4}^{3-}\right]^{2} \longrightarrow (1)\)
given that, somability product \($\left(k_{s p}\right)=8.6 \times 10^{-19}$\)
concentration of \(\mathrm{ca}^{2+}$ ion $=0.0023 \mathrm{M}$\)
putting the value in equation (1) we get,
\($8.6 \times 10^{-19}=(0.0023)^{3} \times\left[\mathrm{PO}_{4}^{3-}\right]^{2}$\)
or, \($\left[\mathrm{PO}_{4}^{3-}\right]^{2}=\frac{8.6 \times 10^{-19}}{(0.0023)^{3}}=7.06 \times 10^{-11}$\)
or, \($\left[\mathrm{PO}_{4}^{3-}\right]=8.40 \times 10^{-6} \mathrm{M}$\)
So the phosphate concentration \($=8.40 \times 10^{-6} \mathrm{M}$\)
What is phosphate concentration ?
Phosphorus is a component of adenosine diphosphate (ADP) and adenosine triphosphate (ATP), both of which utilise the bonds formed between phosphate groups to store energy. Although it is also found in plasma, phosphate is a significant intracellular anion. Adults' typical serum phosphate concentrations fall between\(2.5$ to $4.5 \mathrm{mg} / \mathrm{dL}(0.81$ to $1.45 \mathrm{mmol} / \mathrm{L})$\)So the more about phosphate concentration visit.
https://brainly.com/question/13606926
#SPJ4
the mixing of two particular liquids is an endothermic process. would the formation of this solution be a spontaneous dissolution process?
Answers
The mixing of two particular liquids are spontaneous nonetheless due to the increase in disorder that accompanies formation of the solution.
A spontaneous reaction is one that favours the creation of products under the reaction's current circumstances. An illustration of a spontaneous response is a raging campfire (see illustration below). A fire is exothermic, which implies that when heat is discharged into the environment, the energy of the system decreases. Since gases like carbon dioxide and water vapour make up the majority of a fire's byproducts, the entropy of the system rises during most combustion reactions. Because of this drop in energy and rise in entropy, combustion processes take place on their own.
A nonspontaneous reaction is one that, under the specified conditions, does not favour the creation of products. A driving force or driving factors must favour the reactants over the products for a reaction to be nonspontaneous. In other words, the reaction is endothermic, the entropy is reduced, or both. The majority of the gases that make up our atmosphere are a combination of nitrogen and oxygen. The formation of nitrogen monoxide from these gases might be represented by an equation.
Learn more about Spontaneous:
https://brainly.com/question/30127476
#SPJ4
“What is the group number for the elements that have a stable number of electrons in their outer energy level?”
A. 18
B. 17
C. 1
D. 2
Answers
Answer:
A. 18
Explanation:
Sulfur number sixteen forms a negative
|ω・)
Match each term with the appropriate definition.
spectroscopy
the study of the absorption and emission of
light by atoms
absorption spectrum
represents energy absorbed as dark bands
atomic spectrum
represents energy emitted or absorbed
during electron jumps
emission spectrum
represents energy emitted as colored lines
Answers
Answer:
Spectroscopy — the study of the absorption and emission of light by atoms
Absorption spectrum — represents energy absorbed as dark bands
Atomic spectrum — represents energy emitted or absorbed
during electron jumps
Emission spectrum — represents energy emitted as colored lines
Explanation:
Spectroscopy is an analytical technique to study and analyze using spectrum. It has been the absorption and emission of other light by the atoms. The correct matches are A.1, B. 2, C.3, and D.4.
What is spectroscopy?Spectroscopy is the technique used in chemistry where matter absorbs and emits radiations of a certain wavelength. The absorption spectrum is used to represent absorbed energy in the form of dark bands or lines. Thus, options A.1 and B.2 are correct.
The atomic spectrum is characterized by the black-colored bands that depict the energy absorbed and emitted by an excited atom during electron transitions. Whereas, the emission spectrum shows the colored lines that is the energy emitted when an electron transits from higher to lower energy levels. Thus, options C.3 and D.4 are correct.
Learn more about spectroscopy, here:
https://brainly.com/question/22509226
#SPJ2
What is occurring in the image below?
Melting point elevation
B. Boiling point elevation
C. Freezing point depression
D. Vapor pressure lowering

Answers
The addictive quality is a reduction in vapour pressure.
The vapour pressure at a liquid's normal boiling point is the same as the ordinary atmospheric pressure, which is 1 atmosphere, 760 Torr, 101.325 kPa, or 14.69595 psi.
The pressure that results from liquids evaporating is known as vapour pressure. Surface area, intermolecular forces, and temperature are three often occurring variables that affect vapour press.
lower vapour pressure
raising the boiling point
Low-temperature depression
Osmotic force
They are all dependent on the solute; when you increase the solute, the colligative property and the ratio you added may change.
The Van't Hoff Factor is another option to examine (i). the number of dissolved ions. The colligative property will be further altered if the solute is ionic.
Learn more about Vapor pressure here brainly.com/question/14949391
SPJ1
During the nuclear fission process, the transformation between a parent and daughter isotope involves a a release of neutrons and energy. b an increase in total energy. c the transformation of chemical energy to potential energy. d the transformation of heat energy to kinetic energy.
Answers
Answer:
The energy harnessed in nuclei is released in nuclear reactions. Fission is the splitting of a heavy nucleus into lighter nuclei and fusion is the combining of nuclei to form a bigger and heavier nucleus. The consequence of fission or fusion is the absorption or release of energy.
Introduction
Determine the correct sequence of steps used in carrying out a measurement using a ph probe. Arrange them in order from first to last.
Answers
The correct sequence of steps used in carrying out a measurement using a pH probe is calibration, preparation of the sample, immerse the pH electrode in the sample and recording the pH value.
The steps used in carrying out a measurement using a pH probe are as follows:1. Calibration: The pH electrode must first be calibrated before taking any measurements. The electrodes are calibrated using standard buffer solutions with known pH values.2. Preparation of the sample: Samples must be prepared and placed in a clean container. The sample should be stirred slowly to ensure that the pH is uniform throughout the sample.3. Immerse the pH electrode in the sample: Immerse the pH electrode in the sample and wait for it to stabilize. The electrode must be rinsed with distilled water after each measurement to avoid contamination.4. Recording the pH value: Read the pH value on the display after the pH electrode has stabilized in the sample.
In summary, the pH probe must be calibrated before each measurement, and the sample must be prepared and placed in a clean container. The pH electrode should be immersed in the sample, and the pH value should be recorded after the electrode has stabilized.
To know more about pH probe, click here
https://brainly.com/question/30666605
#SPJ11
Does anyone know how to do this ??

Answers
Answer: you just have to look at the periodic table and fill in the blanks
Explanation:
2. An atom of Be has four protons, five neutrons and four electrons. What 10 poin
is the mas of Be? *
O a.4
b. 5
c. 1
09
Answers
Answer:
Five ( 5 ) is the correct answer
Considering the stereochemistry of the inteediate I below, which of the products would you expect. Explain your answer.
Answers
The expected product is (R)-2-bromobutane.
Stereochemistry plays a crucial role in determining the outcome of chemical reactions. In the given question, the stereochemistry of the intermediate I needs to be considered to determine the expected product.
The intermediate I indicates a chiral carbon center, denoted by an asterisk (*), which means it has four different substituents attached to it. This chiral carbon results in two possible stereoisomers: (R)-2-bromobutane and (S)-2-bromobutane.
When a reaction occurs at a chiral carbon, the stereochemistry of the reactant is usually retained in the product, assuming no racemization or inversion takes place during the reaction. In this case, the intermediate I has an (R) configuration, which implies that the product will also have an (R) configuration.
Therefore, the expected product is (R)-2-bromobutane.
Learn more about (R)-2-bromobutane.
brainly.com/question/17031230
#SPJ11
why does the sky appear orang or red at sunset and sunrise?
Answers
Answer:
Because the sun is low on the horizon, sunlight passes through more air at sunset and sunrise than during the day, when the sun is higher in the sky. More atmosphere means more molecules to scatter the violet and blue light away from your eyes.
be sure to answer all parts. in the electrolysis of a molten mixture of rbf and cacl2, identify the product that forms at the negative electrode and at the positive electrode. negative electrode: rb f2 ca cl2 positive electrode: rb f2 ca cl2
Answers
To answer your question, in the electrolysis of a molten mixture of RbF and CaCl2, the product that forms at the negative electrode is Rb metal and F2 gas.
To answer your question, in the electrolysis of a molten mixture of RbF and CaCl2, the product that forms at the negative electrode is Rb metal and F2 gas. This is because the negative electrode, also known as the cathode, attracts positively charged ions, which in this case is Rb+. The Rb+ ions are reduced by gaining electrons from the cathode and form Rb metal. At the same time, the F- ions in the molten mixture are also attracted to the cathode, and they gain electrons to form F2 gas.
On the other hand, the product that forms at the positive electrode, also known as the anode, is Cl2 gas and Ca metal. This is because the positive electrode attracts negatively charged ions, which in this case is Cl-. The Cl- ions are oxidized by losing electrons at the anode to form Cl2 gas. At the same time, the Ca2+ ions in the molten mixture are also attracted to the anode, and they lose electrons to form Ca metal.
It is important to note that in electrolysis, the cathode is the electrode where reduction occurs, while the anode is the electrode where oxidation occurs. Electrodes are conductive materials that allow the flow of electricity and are used in electrolysis to transfer electrons between the solution and the power source.
To know more about electrolysis visit: https://brainly.com/question/12994141
#SPJ11
Express your answer using two significant figures
What is the temperature of 0.600 mol of gas at a pressure of 1.5 atm and a volume of 11.4 L?
T = ______ K
Answers
The temperature of 0.600 mol of gas at a pressure of 1.5 atm and a volume of 11.4 L is 17 K.
To find the temperature of 0.600 mol of gas at a pressure of 1.5 atm and a volume of 11.4 L, you can use the Ideal Gas Law formula: PV = nRT, where P is pressure, V is volume, n is the number of moles, R is the ideal gas constant, and T is temperature.
Given: P = 1.5 atm, V = 11.4 L, n = 0.600 mol, and R = 0.0821 L atm/mol K.
Rearrange the formula to solve for T:
T = PV/(nR)
T = (1.5 atm × 11.4 L) / (0.600 mol × 0.0821 L atm/mol K)
T = 17.1 K (unrounded)
Expressing the answer using two significant figures, the temperature T = 17 K.
Learn more about temperature: https://brainly.com/question/25290815
#SPJ11
46. Sulfuric acid (H₂SO4) reacts with aqueous sodium cyanide, forming hydrogen cyanide gas and aqueous sodium sulfate.
Answers
Answer:
The balanced chemical equation for the reaction between sulfuric acid (H₂SO4) and aqueous sodium cyanide is:
H₂SO4 + 2 NaCN → 2 HCN + Na₂SO₄
In this reaction, sulfuric acid (H₂SO4) reacts with aqueous sodium cyanide (NaCN) to produce hydrogen cyanide gas (HCN) and aqueous sodium sulfate (Na₂SO₄).
To balance the equation, two moles of sodium cyanide are required for every mole of sulfuric acid. The reaction produces two moles of hydrogen cyanide and one mole of sodium sulfate for every two moles of sodium cyanide and one mole of sulfuric acid.
It's important to note that hydrogen cyanide gas is highly toxic and dangerous, and proper safety precautions must be taken when handling this chemical.
Explanation:
Analyze. This reaction releases energy as heat. Explain whether the reaction is exothermic or endothermic and whether it obeys the laws of Conservation or energy
Answers
Answer:
See explanation
Explanation:
If heat is given out in a reaction, then that reaction is exothermic. If heat is absorbed in a reaction, then that reaction is endothermic.
Hence, if heat is given out, the reaction is exothermic. The heat given out does work on the surrounding thus the laws of conservation of energy are obeyed.
Answer:
The reaction is exothermic.
Explanation:
I took the test
At STP,250cm³of gas had a mass of 0.36g.
1. What result does this give for the molar mass of gas
Answers
As we know STP means Standard Temperature and Pressure. Where, Pressure is 1 atm and temperature is 273 K and also Ideal gas law ( PV=nRT) or (PV = w/M RT)
Where –
P = Pressure in atmV = Volume in Ln = moles R = Ideal gas law constant T = Temperature in Kw = Given MassM = Molar MassNow, according to the question –
w = 0.36 g V = 250 cm³ or \( \sf 250 \times 10^{-3}\) L P = 1 atmT = 273 KR = 0.0821 atm L/ mol KCalculation –
\(\qquad\) \(\pink{\twoheadrightarrow\bf PV = nRT}\)
\(\qquad\) \(\pink{\twoheadrightarrow\bf PV = \dfrac{w}{M}\times RT}\)
\(\qquad\) \(\twoheadrightarrow\sf\dfrac{w}{M} = \dfrac{PV}{RT}\)
\(\qquad\) \(\twoheadrightarrow\sf \dfrac{M}{w} = \dfrac{RT}{PV}\)
\(\qquad\) \(\twoheadrightarrow\sf M = \dfrac{RT}{PV}\times w\)
\(\qquad\) \(\twoheadrightarrow\sf M = \dfrac{0.0821 \times 273 }{1 \times 250\times 10^{-3} } \times 0.36 \)
\(\qquad\) \(\twoheadrightarrow\sf M = \dfrac{22.4}{250\times 10^{-3}} \times 0.36\)
\(\qquad\) \(\twoheadrightarrow\sf M = 89.6\times 0.36\)
\(\qquad\) \(\twoheadrightarrow\sf M = 32.275 \)
\(\qquad\) \(\pink{\twoheadrightarrow\bf M = 32.3\: g}\)
Henceforth, Molar Mass is 32.3 g_______________________________________
The result obtained for the molar mass of the gas is 32.14 g/mol
How to determine the mole of the gas
From the question given above, the following data were obtained:
•Volume (V) = 250 cm³ = 250/1000 = 0.25 L
•Pressure (P) = STP = 1 atm
•Temperature (T) = STP = 273 K
•Gas constant (R) = 0.0821 atm.L/Kmol
•Number of mole (n) =?
The number of mole of the gas can be obtained by using the ideal gas equation as illustrated below:
PV = nRT
Divide both side by RT
n = PV / RT
n = (1 × 0.25) / (0.0821 × 273)
n = 0.0112 mole
How to determine the molar mass of the gasThe number of mole of the gas can be obtained as follow:
•Number of mole of gas = 0.0112 mole
•Mass of gas = 0.36 g
•Molar mass of gas =?
Molar mass = mass / mole
Molar mass of gas = 0.36 / 0.0112
Molar mass of gas = 32.14 g/mol
Learn more about ideal gas equation:
https://brainly.com/question/4147359
Can Chlorine be harmful to any of the earth’s ecosystems? If so how?
Answers
Answer:
Although chlorine itself usually does not cause environmental harm, it combines rapidly to form chemicals such as dioxins that pollute water, contaminate fish and transfer to humans and larger animals that eat the fish.
Explanation:
Enter the ions present in a solution of na2co3 . express your answers as chemical formulas separated by a comma. offset subscripts and charges on each ion; for charges, write the number before the + or - sign.
Answers
The ions present in a solution of Na₂CO₃ are: Na⁺ and CO₃²⁻
Ions are electrically charged particles formed when an atom gains or loses electrons. Atoms that lose electrons become positively charged ions called cations, while atoms that gain electrons become negatively charged ions called anions.
Ions play a crucial role in chemical reactions and the formation of compounds. They can combine together to form ionic compounds through the attraction between opposite charges. In solution, ions are responsible for conducting electricity and participating in various chemical reactions.
Learn more about Ions, here:
https://brainly.com/question/1782326
#SPJ4
Calculate the molecular mass or formula mass (in amu) of each of the following substances: (a) PCl5 amu (b) C4H10 amu (c) NF2 amu (d) Al2O3 amu (e) Fe(NO3)3 amu (f) Mg3N2 amu (g) (NH4)2CO3 amu
Answers
Molecular mass of PCl5 = (1 x atomic mass of P) + (5 x atomic mass of Cl)
Atomic mass of P = 30.97 u
Atomic mass of Cl = 35.45 u
Molecular mass of PCl5 = (1 x 30.97 u) + (5 x 35.45 u) = 208.23 u
(b) C4H10:
Molecular mass of C4H10 = (4 x atomic mass of C) + (10 x atomic mass of H)
Atomic mass of C = 12.01 u
Atomic mass of H = 1.01 u
Molecular mass of C4H10 = (4 x 12.01 u) + (10 x 1.01 u) = 58.12 u
(c) NF2:
Formula mass of NF2 = (1 x atomic mass of N) + (2 x atomic mass of F)
Atomic mass of N = 14.01 u
Atomic mass of F = 18.99 u
Formula mass of NF2 = (1 x 14.01 u) + (2 x 18.99 u) = 52.99 u
(d) Al2O3:
Formula mass of Al2O3 = (2 x atomic mass of Al) + (3 x atomic mass of O)
Atomic mass of Al = 26.98 u
Atomic mass of O = 16.00 u
Formula mass of Al2O3 = (2 x 26.98 u) + (3 x 16.00 u) = 101.96 u
(e) Fe(NO3)3:
Formula mass of Fe(NO3)3 = (1 x atomic mass of Fe) + (3 x (1 x atomic mass of N + 3 x atomic mass of O))
Atomic mass of Fe = 55.85 u
Atomic mass of N = 14.01 u
Atomic mass of O = 16.00 u
Formula mass of Fe(NO3)3 = (1 x 55.85 u) + (3 x (1 x 14.01 u + 3 x 16.00 u)) = 241.99 u
(f) Mg3N2:
Formula mass of Mg3N2 = (3 x atomic mass of Mg) + (2 x atomic mass of N)
Atomic mass of Mg = 24.