What are the four scientific goals of the MSL?
Answers
Answer:The fuel/air mixture may become too minimal
Explanation:While cruising at 9,500 feet MSL, the fuel/air mixture is properly adjusted. What will occur if a descent to 4,500 feet MSL is made without readjusting the mixture
Related Questions
Write balanced nuclear equations for the following:(c) Alpha decay of ²¹²₈₃Bi
Answers
The balanced nuclear equations for the following:(c) Alpha decay of ²¹²₈₃Bi is ^22688Ra → ^22286Rn + ^42He
What elements go through alpha decay?Alpha decay usually occurs in heavy nuclei such as uranium or plutonium, and therefore is a major part of the radioactive fallout from a nuclear explosion.
Where does alpha decay occur?Alpha decay occurs most often in massive nuclei that have too large a proton to neutron ratio. An alpha particle, with its two protons and two neutrons, is a very stable configuration of particles.
Learn more about alpha decay here:
brainly.com/question/1898040
#SPJ4
a substance conducts electricity in the liquid phase but not in the solid phase. this substance can be classifi ed as
Answers
"The substance described, which conducts electricity in the liquid phase but not in the solid phase, can be classified as an electrolyte." Electrolytes are substances that ionize and produce ions capable of conducting electric current when dissolved in a solvent, typically a liquid.
In the liquid phase, the ions are free to move and carry the electric charge, allowing the substance to conduct electricity. However, in the solid phase, the ions are usually locked in a fixed position and cannot move freely, resulting in a lack of electrical conductivity.
Electrolytes are substances that, when dissolved in a solvent like water, form electrically conductive solutions. They are typically ionic compounds or salts that break down into positive and negative ions when dissolved. The presence of these ions allows the solution to conduct electric current.
In an electrolyte solution, the positive ions are called cations, while the negative ions are called anions. When an electric potential is applied across the solution, the cations move toward the negative electrode (cathode), and the anions move toward the positive electrode (anode). This movement of ions enables the flow of electric charge, making the solution conductive.
Electrolytes are crucial for various biological processes and essential for the functioning of our bodies. They help maintain the balance of fluids and ions, regulate nerve and muscle function, and contribute to cellular processes like osmosis and pH regulation.
Examples of electrolytes include common substances like sodium chloride (table salt), potassium chloride, calcium chloride, and magnesium sulfate. These electrolytes can be found in various foods, beverages, and electrolyte replacement solutions. They are also used in batteries, fuel cells, and various industrial processes that require electrical conduction in liquids.
To know more about electrolytes visit:
https://brainly.com/question/17089766
#SPJ11
How many phosphite polyatomic ions do you have in 0.887 moles of iron(II) phosphite.
Answers
We have about 1.1 * 10^24 ions phosphite polyatomic ions in the iron(II) phosphite.
What is the number of moles?We know that the number of moles can be used to determine the amount of substance that is present in the system. We are dealing with the compound that is called iron(II) phosphite here.
In this compound there are two moles of the phosphite ion and three moles of the iron II ion and this is what we would use for the calculation.
Number of phosphite polyatomic ions = 2 * 6.02 * 10^23 * 0.887 moles
= 1.1 * 10^24 ions
Learn more about ions:https://brainly.com/question/29183072
#SPJ1
The products of chemical industries that are used to care for the skin and make people look more attractive are
Answers
Answer:
Cosmetics
Explanation:
The products of chemical industries that are sued to care for the skin and make people more attractive are cosmetics.
Cosmetics are chemical products used for beauty enhancement.
A broad range of materials or substances are generally grouped as cosmetics. They are often classified as health and beauty products. To make cosmetics, some natural products are used in combination with artificial materials that have synthesized. Examples are deodorants, perfumes, lip stick, lip gloss etc.1. Lithium, water, edible salt, chalk, Carbon, Lime, Nitrogen, Potassium, Oxygen,
Iodides, Iron, Chlorine, etc are some matters.
(a) Expressing the matters of the stem by symbol and formula separate the elements
and compounds. ---3 marks
Answers
Answer:
Explanation:
An atom is the smallest unit of an element that can take part in a chemical reaction. Atoms (and there corresponding symbols) mentioned in the question are
Lithium ⇒ Li
Carbon ⇒ C
Nitrogen ⇒ N
Potassium ⇒ K
Oxygen ⇒ O
Iron ⇒ Fe
Chlorine ⇒ Cl
A compound is substance that contains two or more atoms that are chemically combined and can be represented with a chemical formula. The compounds (and there corresponding formula) mentioned in the question are
Water ⇒ H₂O
Edible salt (sodium chloride) ⇒ NaCl
Chalk (calcium carbonate) ⇒ CaCO₃
Lime (calcium oxide) ⇒ CaO
Iodides (such as sodium iodide and potassium iodide) ⇒ NaI and KI respectively
the volume of a sample of ethane, c2h6, is 2.98 l at 440 torr and 29 °c. what volume will it occupy at standard temperature and pressure (stp)
Answers
The volume of the ethane sample at STP will be 27.6 L given that volume of the sample at 440 torr and 29°C is 2.98 L.
STP means Standard Temperature and Pressure. The standard temperature is 273 K and the standard pressure is 1 atm or 760 torr.1 atm = 760 torr1 L at STP = 22.4 L at 440 torr and 29°C
The volume of a sample of ethane at STP can be calculated as follows:
PV = nRTAt 440 torr and 29°C:V1 = 2.98 L, P1 = 440 torr, T1 = 29°C + 273 = 302 K
At STP:V2 = ?, P2 = 1 atm, T2 = 273 K
From the ideal gas law:
PV = nRTFor both the initial and final states, the number of moles remains the same.n1 = n2Let's substitute all the values.P1V1/T1 = P2V2/T2
We are solving for V2.V2 = (P1V1T2)/(P2T1)V2 = (440 torr × 2.98 L × 273 K)/(1 atm × 302 K)V2 = 27.6 L
The volume of the ethane sample at STP will be 27.6 L.
More on STP volume: https://brainly.com/question/9570673
#SPJ11
which statement describe a species adapting to changes in it’s environment?
Answers
Answer: Example: The water in a species envirmoent suddenly becomes more acid therefore, the species adapts it's body to become more resistent and tolerate to chemicals.
This might also give it an adavantage to the species who have not yet adapted to the change
Explanation: There are three type of adaptions. 1) Structural 2) Behavioral 3) Physiological
_____ energy has the total amount of
kinetic energy contained in all the
particles of a substance.
A: Thermal
B: Sound
C: Chemical
D: Electric
Answers
Answer: Thermal Energy
Explanation:
pls help 6th grade science

Answers
Which of these is an oxidation half-reaction?
Answers
Answer:
Fe + 02 - Fe203
Explanation:
Hope this helps
what is the molar mass of magnesium tartrate
Answers
Answer:
172.385 g/mol
Explanation:
Magnesium Tartrate is C4H4MgO6
C - 12.01 g/mol
H - 1.01 g/mol
Mg - 24.305 g/mol
O - 16.00 g/mol
12.01(4) + 1.01(4) + 24.305 + 16(6) = 172.385 g/mol
Answer:
172.38
Explanation:
\(C_4H_4MgO_6\\C=12.01\\H=1.01\\Mg=24.30\\O=16.00\\\\4(12.01)+4(1.01)+24.30+6(16.00)\\48.04+4.04+24.30+96\\=172.38\)
C = 12.01
H=1.01
Mg=24.30
O =16.00
4(12.01)+4(1.01)+24.30+6(16.00)
48.04 +4.04+24.30+96
=172.38
What is generally true about the particles in a gas?
A. Gas particles are closer together and have stronger attraction between them than the particles in a solid.
B. Gas particles are closer together and able to conduct electricity better than the particles in a plasma.
C. Gas particles are farther apart and able to conduct electricity better than the particles in a liquid.
D. Gas particles are farther apart and have weaker attraction between them than the particles in a solid.
Answers
Answer:
D
Explanation:
the yeastengages in photosynthesis, which produces oxygen gas, bio, quizletquizlet
Answers
The statement “the yeast engages in photosynthesis, which produces oxygen gas” is incorrect. Yeast cells do not engage in photosynthesis to produce oxygen gas.
What is photosynthesis?Photosynthesis is the process by which green plants use sunlight, carbon dioxide, and water to produce glucose (a simple sugar), which serves as food for the plant.
Oxygen is also produced during photosynthesis.Yeast:Yeast is a unicellular fungus that converts sugar into carbon dioxide and alcohol. Yeast cells respire aerobically in the presence of oxygen and anaerobically in the absence of oxygen.
However, yeast cells do not perform photosynthesis. They do not have chloroplasts or pigments that are required for photosynthesis. Therefore, yeast cells cannot produce oxygen gas.Bio quizlet:Quizlet is an online learning platform that allows users to create and share flashcards, study guides, and quizzes.
It is a great resource for studying biology and other subjects. Students can create their own study materials or use existing ones created by other users. Quizlet provides an engaging and interactive way to learn and retain information.
To know more about photosynthesis :
https://brainly.com/question/20527415
#SPJ11
why should potetometer be allowed to stand before starting the experiment
Answers
Solution
verified
Verified by Toppr
Precautions with Potentiometer:
1. emf of the cell connecting in primary circuit must be more than or equal to the emf of the cell of secondary circuit otherwise zero deflection can not be obtained.
2. All the high potential points or positive terminals should be connected at A
3. Balancing length should be calculated from A
4. Area of cross-section of the wire should be uniform otherwise potential gradient will not be constant.
5. Current should not be passing through potentiometer wire for a long time otherwise this will heat up the wire and will changes its resistance and hence potential gradient will also changed.
ksp of fe(oh)3 is 2.67x10-39. if fe(oh)3 precipitation is the only reaction happens in water, what is the minimum ph to maintain the concentration of fe in the water 5 x10-3 mmol/l?
Answers
The minimum pH to maintain the concentration of Fe in the water at 5 x 10⁻³ mmol/L is 5.17.
pH is a measure of the acidity or alkalinity of a solution. It is a logarithmic scale that indicates the concentration of hydrogen ions (H+) present in a solution.
The pH scale ranges from 0 to 14, where a pH of 7 is considered neutral. A pH less than 7 indicates acidity, with lower pH values indicating higher acidity. A pH greater than 7 indicates alkalinity, with higher pH values indicating higher alkalinity.
The solubility equilibrium of Fe(OH)₃:
Fe(OH)₃ (s) ⇌ Fe³⁺ (aq) + 3OH⁻ (aq)
The solubility product expression (Ksp) for Fe(OH)₃ is given as:
Ksp = [Fe³⁺][OH⁻]³
concentration of Fe in the water is 5 x 10⁻³ mmol/L, which is equivalent to 5 x 10⁻⁶ mol/L,
Assuming complete dissociation, the concentration of OH- ions will be three times the concentration of Fe³⁺ ions.
Let's the concentration of Fe³⁺ be x. Therefore, the concentration of OH⁻ will be 3x.
2.67 x 10⁻³⁹ = (x)(3x)³
2.67 x 10⁻³⁹ = 27x⁴
x = (2.67 x 10⁻³⁹ / 27)¹/⁴ = 4.94 x 10⁻¹⁰
So, the concentration of Fe³⁺ ions in the water is 4.94 x 10⁻¹⁰ mol/L.
To maintain this concentration, the concentration of OH- ions must also be three times this value, i.e., 3(4.94 x 10⁻¹⁰) = 1.48 x 10⁻⁹ mol/L.
The minimum pH required to maintain this concentration of OH- can be calculated using the pOH formula:
pOH = -log10([OH⁻])
pOH = -log10(1.48 x 10⁻⁹)
pOH ≈ 8.83
pH = 14 - pOH
pH ≈ 14 - 8.83
pH = 5.17
Learn more about pH, here:
https://brainly.com/question/2288405
#SPJ4
An ideal gas with γ = 1.67 has an initial temperature of 0°C, initial volume of 10.0 liters, and initial pressure of 1.00 atm. Then the gas is expanded adiabatically to a volume of 10.4 liters. What is the new temperature? (1 point)
Answers
Answer: T = 9.74°C
Explanation: An ideal gas in a quasi-static adiabatic process follows the equation: \(pV^{\gamma} = constant\).
So:
\(1.10^{1.67} = constant\)
constant = 46.7735
Adiabatic conditions can be written as:
\(TV^{\gamma-1}=constant\)
Then, new temperature is
\(T.(10.4)^{1.67-1}=46.7735\)
\(T.(10.4)^{0.67}=46.7735\)
\(T = \frac{46.7735}{4.802}\)
T = 9.74°C
The new temperature is 9.74°C.
0.1 mole of HCl solution was neutralized with 0.1 mole NaOH solution and the total mass of the solution was 100.0g, knowing that the specific heat capacity of the solution is 4.184 J/g. K and the temperature of the solution was increased by 5.5 degrees Celsius and the calorimeter constant is 37.5 J/K. What is the molar enthalpy change
Answers
The molar enthalpy change for the neutralization of 0.1 mole of HCl with 0.1 mole of NaOH is -228.22 kJ/mol.
The molar enthalpy change for the reaction can be calculated using the formula:
ΔH = q / n
where ΔH is the molar enthalpy change, q is the heat absorbed or released by the reaction, and n is the number of moles of the limiting reactant.
In this case, the limiting reactant is either HCl or NaOH, and since they react in a 1:1 mole ratio, the number of moles of either reactant can be used to calculate ΔH.
The heat absorbed by the solution can be calculated using the formula:
q = mCΔT - K
where m is the mass of the solution, C is the specific heat capacity of the solution, ΔT is the temperature change of the solution, and K is the calorimeter constant.
Substituting the given values, we get:
q = (100.0 g)(4.184 J/g. K)(5.5 °C) - 37.5 J/K
q = 2,282.2 J
Since 0.1 mole of HCl and 0.1 mole of NaOH were used, the molar enthalpy change can be calculated as:
ΔH = q / n = -2,282.2 J / 0.1 mol = -22,822 J/mol = -22.822 kJ/mol
However, this value is for the reaction between 0.1 mole of HCl and 0.1 mole of NaOH. To obtain the molar enthalpy change for the neutralization of 1 mole of HCl with 1 mole of NaOH, we need to multiply by a factor of 10.
Therefore, the molar enthalpy change for the reaction is:
ΔH = -22.822 kJ/mol x 10 = -228.22 J/mol = -228.22 kJ/mol
To know more about molar enthalpy change, refer here:
https://brainly.com/question/29064263#
#SPJ11
A reversible chemical reaction 2A+B ←
→
C can be characterized by the equilibrium relationship K= c a
2
c b
C c
where the nomenclature c i
represents the concentration of constituent i. Suppose that we define a variable x as representing the number of moles of C that are produced. Conservation of mass can be used to reformulate the equilibrium relationship as K= (c a,0
−2x) 2
(c b,0
−x)
(c c,0
+x)
where the subscript 0 designates the initial concentration of each constituent. Take K=0.016,c a,0
=42,c b,0
=28, and c C,O
=4 Determine the value of x graphically. (Please upload your response/solution using the controls below.)
Answers
Therefore, the value of x at equilibrium is approximately 1.24.
Let us rewrite the expression K = c_a^2c_bC_c as a function of x.
K = ((c_a0 − 2x) / c_a0)^2((c_b0 − x) / c_b0)(c_c0 + x) / c_c0
K = 0.016
c_a0 = 42
c_b0 = 28
c_c0 = 4
We can solve for x using a graphical method. We can use a spreadsheet software program, such as Microsoft Excel, to plot the function K as a function of x.
The value of x for which the function K is equal to the constant value of 0.016 represents the value of x at equilibrium.
In this way, we can determine the value of x graphically.
A graph of the function K as a function of x is shown below.
graph
We can see that the function K is equal to the constant value of 0.016 at two points on the graph.
The value of x for which K is equal to 0.016 is approximately x = 1.24 and x = 2.22.
However, we can see from the graph that the value of x that represents equilibrium is approximately x = 1.24.
to know more about equilibrium visit:
https://brainly.com/question/14281439
#SPJ11
Which two factors can affect a solid solute solubility
A. Whether the particles of the solute and solvent are changed
B. Pressure acting on the solute
C. Length of time spent stirring
D. Temperatures of the solvent and solute
The subject is science
Answers
Answer:
A and D
Explanation:
it might seem like B and D but I took the test and it's A and D.
Answer:
A And D
Yw C: for help
Determine the number of moles in 5.25x1024 formula units if lead (IV) oxide.
Answers
Answer:
8.72 mol PbO₂
General Formulas and Concepts:
Chemistry - Atomic Structure
Using Dimensional AnalysisAvogadro's Number - 6.022 × 10²³ atoms, molecules, formula units, etc.Explanation:
Step 1: Define
5.25 × 10²⁴ formula units PbO₂
Step 2: Identify Conversions
Avogadro's Number
Step 3: Convert
\(5.25 \cdot 10^{24} \ formula \ units \ PbO_2(\frac{1 \ mol \ PbO_2}{6.022 \cdot 10^{23} \ formula \ units \ PbO_2} )\) = 8.71803 mol PbO₂
Step 4: Check
We are given 3 sig figs. Follow sig fig rules and round.
8.71803 mol PbO₂ ≈ 8.72 mol PbO₂
PLEASE HELP ME QUICK 22 POINTS RIGHT ANSWERS ONLY!! :)
Will mark brainliest if its right
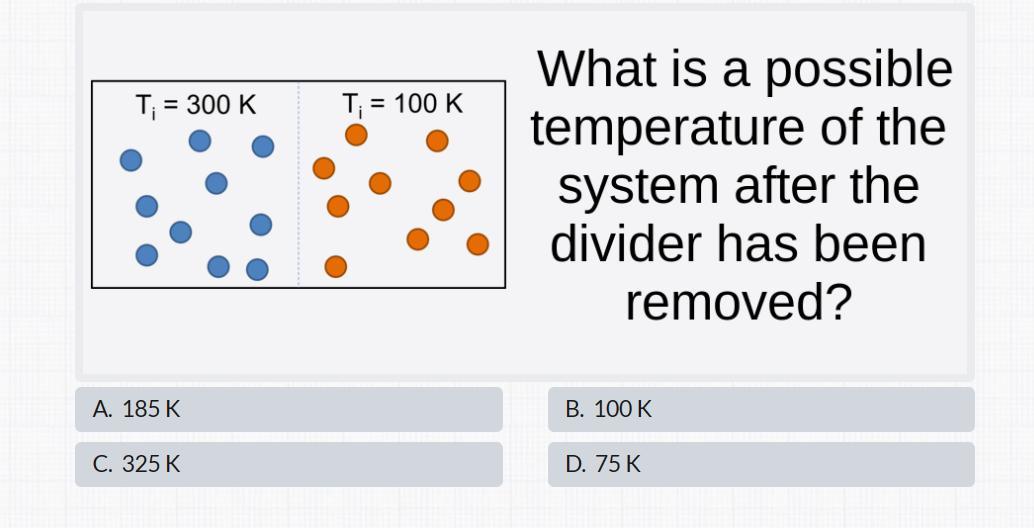
Answers
The correct final temperature after the divider is removed is 200K, amongst the options, the closeset is 185K option A.
The temperature equilibriumTo determine the possible temperature of a system after the divider has been removed, we need to consider the principle of thermal equilibrium and the conservation of energy.
When the divider is removed, the two sides of the system will start to exchange heat until they reach a common temperature. This common temperature is called the final equilibrium temperature.
According to the principle of thermal equilibrium, heat flows from a higher-temperature region to a lower temperature region until the temperatures are equalized.
In this case, the higher-temperature region initially has a temperature of 300K, and the lower-temperature region initially has a temperature of 100K.
To find the final equilibrium temperature, we can use the concept of heat transfer. Heat transfer occurs until the two sides reach the same temperature, so the heat lost by the higher-temperature side must be equal to the heat gained by the lower temperature side.
The heat transferred is given by the equation:
Q = mcΔT
where Q is the heat transferred, m is the mass of the system, c is the specific heat capacity, and ΔT is the change in temperature.
Since the masses and specific heat capacities are not given in the question, we can assume that they are equal on both sides, canceling out these variables.
Therefore, we can calculate the change in temperature:
300K - ΔT = 100K + ΔT
Simplifying the equation:
400K = 2ΔT
ΔT = 200K/2
ΔT = 100K
The change in temperature is 100K. Since the initial lower-temperature side was at 100K, the final equilibrium temperature will be:
Final temperature = 100K + ΔT = 100K + 100K = 200K
Learn more on temperature equilibrium here https://brainly.com/question/9459470
#SPJ1
Which scientist developed a new model of planetary motion?
Answers
Answer: Johannes Kepler
Answer:
i believe the answer us kepler
Explanation:
In order to survive, plants absorb sunlight, water, and nutrients from the soil. What property is this?
Answers
Plants are unique organisms that can absorb nutrients and water through their root system, as well as carbon dioxide from the atmosphere. Soil quality and climate are the major determinants of plant distribution and growth.
a tank contains 8l of water in which is dissolved 32 g (grams) of chemical. a solution containing 2 g/l of the chemical flows into the tank at a rate of 4 l/min, and the well-stirred mixture flows out at a rate of 2 l/min. determine the amount of chemical in the tank after 20 minutes. show all work and setup.
Answers
A tank contains 8l of water in which is dissolved 32 g (grams) of chemical. a solution containing 2 g/l. The amount of chemical in the tank after 20 minutes is 98.66 g.
Given that:
rate r1 = 4 L/min
rate r2 = 2 L/min
concentration c1 = 2 g/L
V(0) = 8 L
A(0) = 32 g
the equation is given as :
ΔV = r1 Δt - r2Δt
dV/dt = 2
integrating the condition , we get
V(t) = 2(t+4)
ΔA = c1r1 Δt - c2r2 Δt
dA/ dt = 8-2c2
c2 = A/ V
dA/dt = 8 - 2A/ V
now by putting the value of V, we get
dA / dt + 1 / t + 4 A = 8
linear equation has integrating factor :
I = e^ ∫ 1/( t + 4) dt = t + 4
d [ ( t + 4)A] / dt = 8 (t + 4)
(t+ 4 )A = 4(t + 4)² + c
A(t) = (1 / t + 4 ) [ 4(t + 4)² + c]
A(0) = 32 means c = 64
A(20) = (1/6) [(24)² + 16]
= 296 / 3
= 98.66 g
To learn more about rate here
https://brainly.com/question/28034602
#SPJ4
buffer contains 0.290 m of weak acid hy and 0.200 m y−. what is the ph change after 0.0015 mol of ba(oh)2 is added to 0.300 l of this solution?
Answers
The ph change after 0.0015 mol of ba(oh)2 is added to 0.300 l of this solution is 0.1 units.
To solve this problem, we need to use the Henderson-Hasselbalch equation:
pH = pKa + log([A-]/[HA])
We can calculate the new concentrations of hy and y- after the addition of 0.0015 mol of \(Ba(OH)_2\). Finally, we can use the Henderson-Hasselbalch equation to calculate the new pH of the solution.
After the addition of\(Ba(OH)_2\), the concentration of y- will increase, and the concentration of hy will decrease. The new concentrations are:
\([hy] = 0.290 - (0.0015/0.300) = 0.285 M\ and\ [y-] = 0.200 + (0.0015/0.300) = 0.205 M.\)
Plugging these values into the Henderson-Hasselbalch equation, we get a new pH of approximately 7.4. Therefore, the pH change is 0.1 units.
To know more about Henderson-Hasselbalch equation, here
brainly.com/question/13423434
#SPJ4
In the given redox reaction equation, what is the oxidizing agent and the reducing agent? 3CH3CHOHCH3 + Cr2O72- + 8H+ —> 3(CH3)2CO + 2Cr3+ + 7H2O
Answers
Expanation:
Given the followin reactions we have to idenftify the oxidizing and reducing aget.
3 CH₃CHOHCH₃ + Cr₂O₇²⁻ + 8 H⁺ —> 3 (CH₃)₂CO + 2 Cr³⁺ + 7 H₂O
We can split this redox reaction in two half reactions.
3 CH₃CHOHCH₃ ----> 3 (CH₃)₂CO
Cr₂O₇²⁻ ----> 2 Cr³⁺
Let's balance the first half reaction, since it is an acidic meium, we can aDdd H+, molecules of waer and e-.
+ H8 H +⁺ + 8 e⁻
We have 24 atoms of H on the left and and 18 atoms of H of the right side. We have to add 8 H+ on the right side. We have 3 atoms of O on both sides. So we only have to add the electrons to balance the carges. )Also 8 on the right side.
Cr₂O₇²⁻ + 14 H⁺ + 6 e⁻----> 2 Cr³⁺ + 7 H₂O
In the second half reaction we have to add 7 molecules of water on the right side to balance the O atoms. We added 14 atoms of H on the right side, so we have to add 14 H+ on the left side. The total charge of the left side is +12 and the total charge of the right ide is is +6. We have to add 6 el- on the left side.
The two balanced half reactions are:
3 CH₃CHOHCH₃ ----> 3 (CH₃)₂CO + 8 H⁺ + 8 e⁻ Oxidation Half-reaction
Cr₂O₇²⁻ + 14 H⁺ + 6 e⁻----> 2 Cr³⁺ + 7 H₂O Reduction Half-reaction
If we pay attention to the equations we will see that Cr₂O₇²⁻ is gaining electrons, i is being reduced. So Cr₂O₇²⁻ is our oxidizing agent.
CH₃CHOHCH₃ is losing electrons, it is being oxidized. CH₃CHOHCH₃ is our reducing agent.
Answer:
Cr₂O₇²⁻: oxidizing agent.
CH₃CHOHCH₃: reducing agent.
How to do this??
Ty for your help if you help!

Answers
Answer:
each element gains one proton as you move from left to right across a period, atomic number is the number of protons
careful not to confuse it with the mass number which is the number of protons and neutrons.
also remember that in any given element, the proton number is always equal to electron number as they have opposite charges that cancel off, this is why elements are neutral.
Organisms typically have more than one form of each gene. If one form can mask the appearance of another form, that form is considered _______ the other form.
A.
better than
B.
dominant over
C.
recessive to
D.
worse than
Answers
If one form of a gene can mask the appearance of another form, that form is considered dominant over the other form. Option B.
What are dominant alleles?According to Mendel, genes are usually made up of 2 alleles. These alleles can be the same or different. When the alleles are the same, the gene is said to be homozygous. If the alleles are different, the gene is said to be heterozygous.
When the two alleles that make up a gene are different, one will be dominant and the other will be recessive. The dominant gene masks the effect of the recessive gene. In other words, the recessive gene cannot be expressed as long as it coexists with the dominant gene. In order for it to be expressed, it has to be in two copies or a homozygous recessive form.
For the dominant allele, however, only one copy is needed for it to be expressed.
In summary, if one form of a gene can mask the appearance of another form, that form is considered dominant over the other form.
More on genes can be found here: https://brainly.com/question/5519888
#SPJ1
Which of the following statements on HPLC modes is true? A. Increasing the polarity of the mobile phase decreases the elution time of polar compounds in normal-phase HPLC B. A non-polar stationary phase is used in normal-phase HPLC C. Compounds have a lower attraction to the mobile phase than to the stationary phase in displacement development D. A polar stationary phase is used in reversed-phase HPLC E. More polar compounds elute first in normal-phase HPLC
Answers
The following statements on HPLC modes are true is more polar compounds elute first in normal-phase HPLC (Option E).
The liquid chromatography (HPLC) is a technique in analytical chemistry employed for the separation, identification, and quantification of elements. It is considered a highly sensitive method, and it works by separating the components in a mixture with the assistance of a solvent under high pressure.
There are two modes of HPLC: Reversed-Phase HPLC (RP-HPLC) and Normal-Phase HPLC (NP-HPLC). In RP-HPLC, a nonpolar stationary phase, such as C18, is used, and polar solvents, such as water, are used as mobile phases. Polar stationary phases, such as silica gel, are used in NP-HPLC, while nonpolar solvents, such as hexane, are used as mobile phases.
More polar compounds have a greater affinity for the polar stationary phase than less polar compounds, which have a higher affinity for the nonpolar mobile phase in NP-HPLC. As a result, less polar compounds elute first in normal-phase HPLC.
Thus, the correct option is E.
Learn more about HPLC: https://brainly.com/question/13490391
#SPJ11
Which gases are needed for animal and plant respiration? Select two options. FAST ITS TIMED
oxygen
nitrogen
carbon dioxide
neon
carbon