What are the four processes of the Rankine Cycle?
Answers
Isentropic compression; Constant-pressure heat addition; Isentropic expansion; Constant-pressure heat rejection.
Explanation:
The four processes of the Rankine Cycle are:
1. Isentropic compression: In this process, the working fluid (usually water) is compressed in a pump, increasing its pressure but maintaining its entropy.
2. Constant-pressure heat addition: The compressed fluid then enters a boiler where it is heated at constant pressure, turning it into superheated steam.
3. Isentropic expansion: The superheated steam expands in a turbine, where it does work on the turbine blades, converting its thermal energy into mechanical work. During this process, the steam's pressure and temperature decrease while its entropy remains constant.
4. Constant-pressure heat rejection: Finally, the expanded steam enters a condenser, where it releases heat at constant pressure and condenses back into liquid water. The water then returns to the pump, and the cycle begins again.
These four processes together constitute the Rankine Cycle, which is widely used in power generation and refrigeration systems.
To know more about Rankine Cycle:
https://brainly.com/question/16836203?
#SPJ11
Related Questions
A study was conducted of 90 adult male patients following a new treatment for congestive heart failure. One of the variables measured on the patients was the increase in exercise capacity (in minutes) over a 4-week treatment period. The previous treatment regime had produced an average increase of μ=2 minutes. The researchers wanted to evaluate whether the new treatment had increased the value of μ in comparison to the previous treatment. The data yielded y(bar)=2.17 and s=1.05.
(a) if the actual value of mu is 2.1 and alpha is reduced from 0.05 to 0.01, what would be the effect on the power curve?
(b) If the sample size is reduced from 90 to 50, what would be the effect on the power curve?
Answers
a. Decreasing alpha from 0.05 to 0.01 makes the significance level more stringent. You will be less likely to reject the null hypothesis, even when it's false. This increases the probability of a Type II error, thus potentially reducing the power of the test. The power curve will shift to the left.
b. If the sample size is reduced from 90 to 50, the effect on the power curve is that it will also shift towards the left.
What more should you know about decreasing the alpha and the power curve?The power curve is a graph that shows the probability of rejecting the null hypothesis as a function of the true value of the mean.
In the given scenarios of this study, Reducing the significance level and reducing the sample size will shift the power curve to the left, indicating a decrease in the statistical power of the test.
The power of a statistical test is the probability that it correctly rejects the null hypothesis when the alternative hypothesis is true.
a) Reducing alpha from 0.05 to 0.01 means that we are more stringent in our assessment of whether the new treatment is effective.
This will result in a decrease in the power of the test, meaning that it is less likely that we will be able to detect a difference between the new treatment and the previous treatment.
b) If the sample size is reduced from 90 to 50, the effect on the power curve is that it will also shift towards the left.
This is because a smaller sample size decreases the power of the test. A larger sample size provides more information and thus makes it more likely to correctly reject the null hypothesis when the alternative hypothesis is true.
Therefore, by reducing the sample size, you are decreasing the likelihood of detecting a true effect if one exists, thus reducing the power of the test.
Find more exercises on alpha level in a study;
https://brainly.com/question/6372035
#SPJ4
5. What is the difference between a reflected and a diffracted sound wave?
A. A reflected sound wave is absorbed by the medium. A diffracted sound wave changes
direction as it passes through an opening.
B. A diffracted sound wave bounces back to the place of origin. A reflected sound wave is
absorbed as it passes through an opening.
C. A reflected sound wave bounces back to the place of origin. A diffracted sound wave
changes direction as it passes through an opening.
D. A diffracted sound wave bounces back to the place of origin. A reflected sound wave
changes direction as it passes through an opening.
Answers
Answer:
Reflection involves a change in direction of waves when they bounce off a barrier; refraction of waves involves a change in the direction of waves as they pass from one medium to another; and diffraction involves a change in direction of waves as they pass through an opening or around a barrier in their path.
Explanation:
https://www.physicsclassroom.com/class/waves/Lesson-3/Reflection,-Refraction,-and-Diffraction#:~:text=Diffraction%20of%20Waves-,Reflection%20involves%20a%20change%20in%20direction%20of%20waves%20when%20they,a%20barrier%20in%20their%20path.
Reflection of sound wave differs from diffraction of sound wave in that a reflected sound wave bounces back to the place of origin while a diffracted sound wave changes direction as it passes through an opening.
What is reflection?Reflection is the bouncing back of a wave when it hits an obstacle along its path.
What is diffraction?Diffraction is the spreading out of a wave in a circular path as it passes through a tiny opening.
Therefore, the difference between reflection and diffraction is that a reflected sound wave bounces back to the place of origin while a diffracted sound wave changes direction as it passes through an opening.
Learn more about reflection and diffraction at: https://brainly.com/question/11176463
Identify how many of the original parent nuclei remain after three half-lives
Answers
Answer:
yes
Explanation:
i did the test
That's just the tip of the iceberg" is a popular expression you may have heard. It means that what you can see is only a small part of the overall problem. As the diagram shows, most of an iceberg is actually out of sight, below the water level. Based on this diagram, what is the most likely density of the iceberg? (Assume a density of 1.03 g/mL for seawater.)
A. 0.88 g/cc
B. 1.23 g/cc
C. 0.23 g/cc
D. 4.14 g/cc
Answers
B 1.23 g/cc
Explanation:
For something to float on seawater, the density must be less than 1.03 g/mL. If the object sinks, the density is greater than 1.03 g/mL.
Let’s examine the answer choices. Keep in mind, the ice berg is mostly below the water level.
A. 0.88 g/cc
This is less than 1.03 g/cc, which would result in floating.
B. 1.23 g/cc
This is the best answer choice. The iceberg is mostly beneath the water, but some of it is exposed. The density is greater than 1.03 g/mL, but not so much greater that it would immediately sink.
C. 0.23 g/cc
This is less than 1.03 g/cc, which would produce floating.
D. 4.14 g/cc
This is much greater than 1.03 g/cc and the result would be sinking.
Write word equation of carbon burns in oxygen
Answers
Answer:
Word equation carbon + oxygen → carbon dioxide
Chemical equation C + O2 → CO2
1. The author says that bog bodies were discovered as long ago as the 1600s, but the only ones existing today are those found after the late 1800s. What hap- pened to the earlier bog bodies?
Answers
Answer:
The earlier bog bodies that were discovered in the 1600s might have not been preserved properly due to a lack of knowledge on how to preserve them or a lack of awareness of their significance. It is also possible that they might have decayed and decomposed over time and not survived till the present day. However, the bog bodies found after the late 1800s were preserved and studied extensively due to the increasing awareness and understanding of their historical and archaeological significance.
Explanation:
Hope this helped!! Have a great day/night!!
This organelle is like the border patrol because it determines what can go into and out of the cell.
Answers
The organelle that is responsible for controlling what enters and exits the cell is the cell membrane. It is like a border patrol in that it acts as a barrier that determines what can pass through it and what cannot.
The cell membrane is made up of a phospholipid bilayer with embedded proteins and carbohydrates that help regulate the movement of molecules in and out of the cell. In summary, the long answer to your question is that the cell membrane is the organelle that acts as a border patrol, determining what can go into and out of the cell.
The organelle that acts like the border patrol for a cell is the cell membrane. It determines what can go in and out of the cell, ensuring proper regulation of materials and maintaining the cell's internal environment.
To know more about cell membrane visit:-
https://brainly.com/question/13524386
#SPJ11
determine how much heat (in kj) of 2.89 mol of tio2(s)
Answers
Total heat generated by 2 mole of TiO2(s) is 4.963kJ.
The amount of heat released in the reaction of 2.89 mol of TiO2(s) can be calculated using the following equation: q = nCΔT, where n is the number of moles, C is the specific heat capacity of TiO2, and ΔT is the change in temperature.
The specific heat capacity of TiO2 is 683. 697. J/kgK. and the change in temperature is is 25k. By plugging in the values and converting J to kJ,
q = 2.89 * 25 * 683.697
=> 4963.35
In brief, the amount of heat released by 2.89 mol of TiO2(s) is 4.963kJ.
To know more about specific heat capacity click on below link:
https://brainly.com/question/16952828#
#SPJ11
Complete question :
determine how much heat (in kj) of 2.89 mol of tio2(s) with a temperature difference of 25k
How many moles are in 6.243 x 10^24 molecules of H2O
Answers
Taking into account the definition of Avogadro's Number, 10.365 moles of H₂O are 6.243×10²⁴ molecules of H₂O.
Definition of Avogadro's NumberAvogadro's Numberis called the number of particles that make up a substance (usually atoms or molecules) and that can be found in the amount of one mole of said substance.
Its value is 6.023×10²³ particles per mole and it applies to any substance.
Number of moles of H₂OYou can apply the following rule of three: if 6.023×10²³ molecules are contained in 1 mole of H₂O, then 6.243×10²⁴ molecules are contained in how many moles of H₂O?
amount of moles of H₂O= (6.243×10²⁴ molecules× 1 mole)÷ 6.023×10²³ molecules
amount of moles of H₂O= 10.365 moles
Finally, 10.365 moles of H₂O are 6.243×10²⁴ molecules of H₂O.
Learn more about Avogadro's Number:
brainly.com/question/11907018
#SPJ1
HELP ME ANSWER THIS
If you could somehow travel inside an atom and look around, what part of the atom would you want to look at? Why?
Answers
Answer:
I'd wanna see whats in the nuclues
Explanation:
Because its interesting to know what and how it really looks like up close. I also wanna know if an electron really has a light mass
please respond and help if you’re able to

Answers
f 3.71 mol of an ideal gas has a pressure of 2.15 atm and a volume of 64.37 l, what is the temperature of the sample in degrees celsius?
Answers
The temperature of the ideal gas sample is -104.8 °C.
To find the temperature of the ideal gas sample, we can use the ideal gas law,
PV = nRT
where P is the pressure, V is the volume, n is the number of moles, R is the gas constant, and T is the temperature in Kelvin.
T = PV/nR
T = (2.15 atm) x (64.37 L) / (3.71 mol x 0.08206 L·atm/mol·K)
T = 168.4 K
To convert from Kelvin to Celsius, subtract 273.15:
T = -104.8 °C
Therefore, the temperature of the sample is -104.8 °C. The result is a negative temperature, which indicates that the gas is very cold.
To know more about the ideal gas, here
brainly.com/question/15315303
#SPJ4
Experiment 1: in the synthesis reaction, the white powder produced is magnesium oxide (mgo, mm = 40.3 g/mol). how many moles (n) of magnesium oxide were formed? select the closest answer.
Answers
1.98 moles of Magnesium oxide were formed.
What is Balanced Chemical Equation ?The balanced chemical equation is the equation in which the number of atoms on the reactant side is equal to the number of atoms on the product side in an equation.
Now write the balanced chemical equation
2Mg + O₂ → 2MgO
(2 × 24) 2 × (24 + 16)
48 g of Mg gives 80 g of MgO
How to find the number of moles ?To find the number of moles use the expression
Number of moles = \(\frac{\text{Given mass}}{\text{Molar mass}}\)
= \(\frac{80}{40.3}\)
= 1.98 mole
Thus from the above conclusion we can say that 1.98 moles of Magnesium oxide were formed.
Learn more about the Balanced Chemical Equation here: brainly.com/question/26694427
#SPJ1
How much calcium oxide would be made by the thermal decomposition of 25 grams of calcium carbonate?
CaCO3 -> CaO + CO2

A. 28 grams
B. 12 grams
C. 14 grams
D. 25 grams
Answers
Answer:
14 grams of calcium oxide would be produced by thermal decomposition of 25 grams of calcium carbonate.
Explanation:
You know:
CaCO₃ → CaO + CO₂
In the first place, by stoichiometry of the reaction (that is, the relationship between the amount of reagents and products in a chemical reaction) the following quantities react and are produced:
CaCO₃: 1 moleCaO: 1 moleCO₂: 1 moleBeing:
Ca: 40 g/moleC: 12 g/moleO: 16 g/molethe molar mass of the compounds participating in the reaction is:
CaCO₃: 40 g/mole + 12 g/mole + 3*16 g/mole= 100 g/moleCaO: 40 g/mole + 16 g/mole= 56 g/moleCO₂: 12 g/mole + 2*16 g/mole= 44 g/moleThen, by stoichiometry of the reaction, the following mass amounts of the compounds participating in the reaction react and are produced:
CaCO₃: 1 mole* 100 g/mole= 100 gCaO: 1 mole* 56 g/mole= 56 gCO₂: 1 mole* 44 g/mole= 44 gYou can then apply the following rule of three: if by stoichiometry of the reaction 100 grams of calcium carbonate CaCO₃ produce 56 grams of calcium oxide CaO, 25 grams of CaCO₃ how much mass of CaO will it produce?
\(mass of calcium oxide=\frac{25 grams of CaCO_{3} *56 grams of CaO}{100 grams of CaCO_{3} }\)
mass of calcium oxide= 14 grams
14 grams of calcium oxide would be produced by thermal decomposition of 25 grams of calcium carbonate.
What is the [H+] if the pH of a solution is 3.20?
(in scientific notation)
Answers
Answer:
[H⁺] = \(6.3096\)×\(10^{-4}\) M
Explanation:
pH= - log[H⁺]
=> 3.2 = - log[H⁺]
=> [H⁺] = \(10^{-3.2}\)
∴ [H⁺] = \(6.3096\)×\(10^{-4}\) M
pls mark it as brainliest
show that the following language is decidable: {〈g〉 : g is a cfg and there exists a string that is in l(g) and has at least one a terminal} hint: modify the algorithm for ecfg
Answers
The language is decidable.
{〈g〉 : g is a CFG and there exists a string that is in L(G) and has at least one a terminal}.
algorithm to decide the language:
Given: Language is
{〈g〉 : g is a CFG and there exists a string that is in L(G) and has at least one a terminal}.
We need to show that the language is decidable. Let L be a context-free language generated by a CFG
G = (V, T, P, S).
We have to decide whether there exists at least one string in L which contains at least one 'a' terminal. Let S1 be a new start symbol with a production rule of the form S1 → S. We can add a new terminal symbol 'b' which is not present in the original grammar. We can also add new production rules as follows:
S1 → S|bS → a|b|Sa|SS|AS|BBSS → a|b|Sa|SS|AS|BBAS → a|b|Sa|SS|AS|BBBB → a|b|Sa|SS|AS|BB|ε
The following is the algorithm to decide the language.
1. Input: Context-free grammar G.
2. Construct a new grammar G' from G using the above production rules.
3. Construct the CYK table for all strings of length 1 to n, where n is the length of the longest string in the grammar.
4. If there exists a cell in the CYK table such that it contains S1 and a terminal 'a', then the language generated by G contains at least one string which has at least one 'a' terminal. Otherwise, the language generated by G does not contain any string which has at least one 'a' terminal.
5. Halt.
The language is decidable.
To know more about algorithm visit:
https://brainly.com/question/28724722
#SPJ11
Is mixing salt and pepper physical change or chemical change ?
Answers
Answer:Cutting, tearing, shattering, grinding, and mixing are further types of physical changes because they change the form but not the composition of a material. For example, mixing salt and pepper creates a new substance without changing the chemical makeup of either component.
Explanation:
A sample of gas is in a container with a fixed volume. a student notices that as the temperature increases so does the pressure. this is a(n) relationship.
Answers
direct relationship
When the temperature increases and so does the pressure, it is a direct relationship. It denotes direct proportionality.
Gay Lussac's Law states that:
"The pressure of a given amount of gas held at constant volume is directly proportional to the Kelvin temperature."
What does the law of Gay Lussac explain?An unchanging volume of gas is present in an inflated tire. Due to Gay Lussac's law, the pressure of the gas inside the tire rises in the summer when the temperature is high. The pressure inside the tires will rise as the temperature rises, and at a certain point, the tires will fracture.How is the law of Gay Lussac applied in practice?The rupture of a pressure cooker, an aerosol can, and a tire are a few examples of Gay-Lussac's law occurring in real life. When these compounds are exposed to higher temperatures, they all detonate. Gay-Lussac's Law explains the explosion's underlying scientific cause.To learn more about Gay-Lussac's law visit:
https://brainly.com/question/2683502
#SPJ4
Octane has a density of 0.702 g slash centimeters cubed what is the mass of 32 cm³ of octane
Answers
\(\\ \rm\longmapsto Density=\dfrac{Mass}{Volume}\)
\(\\ \rm\longmapsto Mass=Density\times Volume\)
\(\\ \rm\longmapsto Mass=0.702(32)\)
\(\\ \rm\longmapsto Mass=22.464g\)
when does the system in the graph reach equilibrium? pleaseeee help fast!!
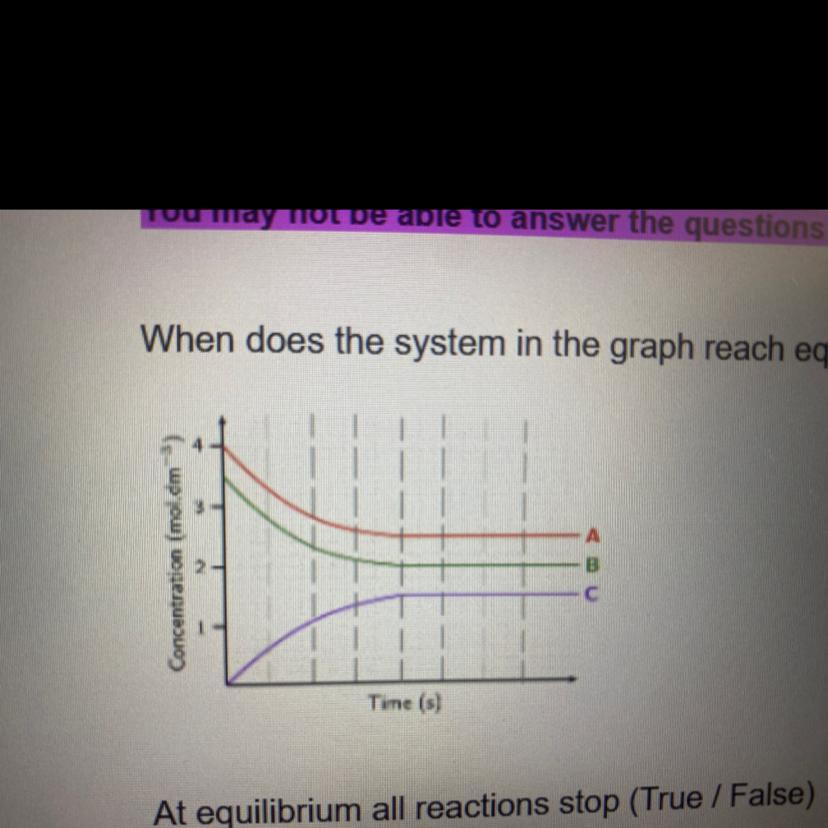
Answers
The system is now in equilibrium when both the forward reaction rate and the reverse reaction rate are equal. When the concentration and number of moles of a products and reactants are constant.
How does an ecological system come to balance?Whenever the rates of the forward or reverse reactions are equal, a system is said to be in equilibrium. The pace of the ahead reaction rises with the addition of more reactants. It appears that the equilibrium is shifting towards the product, and right, part of the equation since the rate of a reverse reaction remains unchanged at first.
How can equilibrium be determined?When there is no trend for the amounts of reactants and byproducts to fluctuate, a chemical process is in equilibrium. They both produce the same mixture all components after the transition is complete, and they both represent the very same chemical reaction process in which the components' functions are reversed.
To know more about moles visit:
https://brainly.com/question/26416088
#SPJ1
the potential energy of a pair of hydrogen atoms separated by a large distance x is given by u(x)=−c6/x6 , where c6 is a positive constant.
Answers
The potential energy (u) of a pair of hydrogen atoms separated by a large distance x is given by the equation = u(x) = -c6/x^6. The equation provided represents a simplified model of the interaction between hydrogen atoms, known as the Lennard-Jones potential.
In this equation, c6 is a positive constant that determines the strength of the interaction between the hydrogen atoms. The potential energy is inversely proportional to the sixth power of the distance between the atoms.
As the distance (x) between the hydrogen atoms increases, the potential energy decreases rapidly. This is because the negative sign indicates an attractive force between the atoms. The potential energy approaches zero as the distance between the atoms becomes very large.
Conversely, as the distance between the hydrogen atoms decreases, the potential energy becomes more negative, indicating a stronger attractive force between the atoms.
In reality, the interaction between atoms is more complex and involves other factors such as electron-electron repulsion and electron-nucleus attraction.
To know more about hydrogen atom
https://brainly.com/question/24433860
#SPJ11
Why do the voices sound different when you listen to them through the wall?
Sound waves travel at different speeds when traveling through different
materials
Sound waves change frequency over long distances
Sound waves do not pass through solid materials
Your ear processes sound differently when it goes through a solid.
Answers
Answer:
Sound waves travel at different speeds when traveling through different materials
Explanation:
This is because in water the particles are more closely packed together, therefore making the sound travel faster and sound louder. The opposite is the same for air.
What would be the charge of an atom that has lost 3 electrons?
Answers
The 3+ charge next to the symbol indicates a loss of 3 electrons
When atoms form ions, they lose or gain electrons. The nucleus remains intact; there is no change in the number of protons and neutrons. A loss of electrons produces a positively charged ion (a cation).
Ex= As aluminium lost 3 electrons, its ion will have a 3+ charge since the ion has 3 more positively charged protons than negatively charged electrons (ie, 13 protons, 10 electrons).
Atoms of elements which lose electrons develop a positive charge, such as the aluminium ion, Al3+ , which results when an aluminium atom loses three electrons; or the magnesium ion, Mg2+ , which results when a magnesium atom loses two electrons.
To know more about Atom click here
https://brainly.com/question/17545314
#SPJ4
A sample of neon gas was collected into a 1.50 L flask and was found to have a pressure of 675 mmHg. If this sample of gas is transferred to a container with a volume of 0.425 L what is the new pressure (in mmHg)
Answers
The new pressure of the neon gas sample in the container with a volume of 0.425 L is 2385 mmHg.
To solve this problem, we can use the combined gas law, which states:
(P₁ * V₁) / T₁ = (P₂ * V₂) / T₂
Where:
P₁ and P₂ are the initial and final pressures respectively,
V₁ and V₂ are the initial and final volumes respectively,
T₁ and T₂ are the initial and final temperatures respectively.
In this case, the temperature remains constant, so we can remove it from the equation. We can rearrange the formula to solve for P₂:
P₂ = (P₁ * V₁) / V₂
Given:
P₁ = 675 mmHg
V₁ = 1.50 L
V₂ = 0.425 L
Substituting the values into the equation:
P₂ = (675 mmHg * 1.50 L) / 0.425 L
P₂ = 2385 mmHg
Therefore, the new pressure of the neon gas sample in the container with a volume of 0.425 L is 2385 mmHg.
Know more about pressure here,
https://brainly.com/question/29341536
#SPJ11
the individual thermodynamic contribution of w (rm)chain a was found to increase the interaction energy of the mkr681h dimer. if so, what must be true for chain a?
Answers
The individual thermodynamic contribution of w(rm)chain that was found to be increase the interaction energy of the MKR681H dimer. if so, The ture for the chain A is ΔGsolv < 0. The option A is correct.
The expression is as :
W (RM)int = W (RM)dimer - W (RM)chain A - W (RM)chain B
If the W (RM)chain A will increases the interaction energy for the MKR681H dimer, the W (RM)int, the W (RM)chain A must be the negative quantity, Like that the -W (RM)chain A term in the above equation becomes the positive value.
If the W (RM)chain A < 0, then the one or the both of the terms H intra and the ΔGsolv must be negative. Therefore, the option A is correct.
To learn more about thermodynamic here
https://brainly.com/question/20114432
#SPJ4
This question is incomplete, the complete question is :
The individual thermodynamic contribution of W (RM)chain A was found to increase the interaction energy of the MKR681H dimer. If so, what must be true for chain A?
A. ΔGsolv < 0
B. ΔGsolv = 0
C. W (RM)chain A > 0
D. Hintra < 0
Solutions of Ag , Cu2 , Fe3 and Ti4 are electrolyzed with a constant current until0.10 mol of metal is deposited. Which will require the greatest length of time
Answers
Ti4+ will require the greatest length of time for electrolysis as it requires the transfer of the greatest number of electrons.
To determine which solution will require the greatest length of time for 0.10 mol of metal to be deposited, we need to consider the number of electrons involved in the reduction reactions of each metal ion.
For Ag, Cu2, Fe3, and Ti4, the respective reduction reactions are:
Ag+ + e- → Ag (1 electron)
Cu2+ + 2e- → Cu (2 electrons)
Fe3+ + 3e- → Fe (3 electrons)
Ti4+ + 4e- → Ti (4 electrons)
Since Ti4+ requires the most electrons (4) for reduction, it will take the longest time to deposit 0.10 mol of metal when electrolyzed with a constant current.
To know more about electrolysis click on below link :
https://brainly.com/question/12054569#
#SPJ11
Answer:
Ti4+ will require the greatest length of time for the deposition of 0.10 mol of metal using a constant current.
Explanation:
The time required for the deposition of 0.10 mol of metal will depend on the current and the number of electrons required for the reduction of each metal ion. The time can be calculated using Faraday's law, which relates the amount of electric charge passed through a solution (in coulombs) to the amount of substance produced or consumed during an electrolysis reaction.
The equation for Faraday's law is:
Q = nF
where Q is the amount of electric charge (in coulombs), n is the number of moles of substance produced or consumed, and F is the t Faraday constant (96,500 C/mol).
The number of electrons required for the reduction of each metal ion can be determined from the balanced half-reaction for each metal:
Ag+ + e- → Ag (1 electron)
Cu2+ + 2e- → Cu (2 electrons)
Fe3+ + 3e- → Fe (3 electrons)
Ti4+ + 4e- → Ti (4 electrons)
Using the above information, we can calculate the time required for the deposition of 0.10 mol of metal using a constant current. Assuming a current of 1 ampere (1 C/s), the time required for each metal is:
Ag: Q = nF = (0.10 mol)(96,500 C/mol) = 9,650 C
t = Q/I = 9,650 C / 1 A = 9,650 s = 2.68 hours
Cu: Q = nF = (0.10 mol)(2)(96,500 C/mol) = 19,300 C
t = Q/I = 19,300 C / 1 A = 19,300 s = 5.36 hours
Fe: Q = nF = (0.10 mol)(3)(96,500 C/mol) = 28,950 C
t = Q/I = 28,950 C / 1 A = 28,950 s = 8.04 hours
Ti: Q = nF = (0.10 mol)(4)(96,500 C/mol) = 38,600 C
t = Q/I = 38,600 C / 1 A = 38,600 s = 10.72 hours
TO KNOW MORE ON Faraday's law REFER,
https://brainly.com/question/1640558#
#SPJ11
What physical property can be used to distinguish between a 1 cm cube of copper
and a 1 cm cube of sugar?
Select one:
a size
b. lustre
C. cost
d state
Answers
State physical property can be used to distinguish between a 1 cm cube of copper and a 1 cm cube of sugar. Hence, option D is correct.
What is physical property?A property (such as colour, hardness, boiling point) of the matter is not involved in its manifestation of a chemical change.
Physical properties are those general properties you notice most readily about a substance, such as its size, state of matter (solid, liquid, or gas), colour, mass, density and strength.
Values for physical properties can be determined by tests that don't alter the substance being tested.
Hence, state physical property can be used to distinguish between a 1 cm cube of copper and a 1 cm cube of sugar.
Learn more about the physical property here:
https://brainly.com/question/18327661
#SPJ2
What geographic obstacle causes water to condense in an air mass as the air mass moves? Explain your answer.
A. Forests
B.oceans
C.prairies
D.mountains
Answers
Answer:
Mountains
Explanation:
Mountains are colder than the lower elevations. When humid air enters a cooler region, the saturated air begins to cool and this reduces the ability of the air to maintain the evaporated water is the gas state. Molecules of water have reduced enerfy at the cooler temperatures. They will slow down and coalesce with other water molecules to form small droplets of water. The atmoshere cannot hold these heaevier droplets and they begin to fall (i.e., rain).
One migh ask why does the air temperature drop at higher altitudes? Heat rises, so why shouldn't it be warmer on top a mountain, compared to the valley. The reason is that the atmosphere becomes less dense the further from Earth's center on gets. This less dense air has a lower water saturation point, so it rains.
Why is an atom considered electrically neutral?
Answers
Explanation:
A proton and an electron have an equal amount but an opposite type of charge. Thus, if an atom contains equal numbers of protons and electrons, the atom is described as being electrically neutral.
Answer:
.
Explanation:
A proton and an electron have an equal amount but an opposite type of charge. Thus, if an atom contains equal numbers of protons and electrons, the atom is described as being electrically neutral.
Position always is relative to another object or location
options: True False
Answers
Answer:
True
Explanation:because of the motion