What are requirements before you can compare the energy (or enthalpy or Gibbs free energy) for 2 compounds? (choose all that apply) a Both compounds must have had a geometry optimization b They must have the same formula c Both compounds must have been calculated at the same level of theory
Answers
The requirements before you can compare the energy (or enthalpy or Gibbs free energy) for 2 compounds are both compounds must have had a geometry optimization, and both compounds must have been calculated at the same level of theory.
Geometry optimization ensures that the compounds are in their most stable state, which allows for a fair comparison of their energies. Calculating both compounds at the same level of theory ensures that the comparison is consistent and the results are reliable, as different levels of theory can produce varying results.
To accurately compare the energy, enthalpy, or Gibbs free energy of two compounds, they must have undergone geometry optimization and be calculated at the same level of theory. Having the same formula is not a requirement for comparison.
To know more about energy, click here
https://brainly.com/question/1932868
#SPJ11
Related Questions
amy combined 10.0 ml of 1.0 m hcl with 5.0 ml of 2.0 m naoh. will the reaction have reached its equivalence point once the reaction is complete?
Answers
The reaction has reached its equivalence point once it is complete.
To determine if the reaction has reached its equivalence point once the reaction is complete, we must first calculate the moles of each compound:
HCl moles = 1.0 M x (10.0 mL / 1000 mL/L) = 0.01 mol
NaOH moles = 2.0 M x (5.0 mL / 1000 mL/L) = 0.01 mol
The two compounds react in a 1:1 ratio.
There are now no more moles of HCl or NaOH left to react since they have equal moles.
We can thus conclude that the reaction has reached its equivalence point as soon as the reaction is over. Since the moles of both HCl and NaOH have been completely neutralized, the pH at the equivalence point is 7.
This indicates that the reaction has reached its equivalence point once it has finished.
To learn more about equivalence point refer - https://brainly.com/question/29385269
#SPJ11
How much calcium oxide would be made by the thermal decomposition of 25 grams of calcium carbonate?
CaCO3 -> CaO + CO2

A. 28 grams
B. 12 grams
C. 14 grams
D. 25 grams
Answers
Answer:
14 grams of calcium oxide would be produced by thermal decomposition of 25 grams of calcium carbonate.
Explanation:
You know:
CaCO₃ → CaO + CO₂
In the first place, by stoichiometry of the reaction (that is, the relationship between the amount of reagents and products in a chemical reaction) the following quantities react and are produced:
CaCO₃: 1 moleCaO: 1 moleCO₂: 1 moleBeing:
Ca: 40 g/moleC: 12 g/moleO: 16 g/molethe molar mass of the compounds participating in the reaction is:
CaCO₃: 40 g/mole + 12 g/mole + 3*16 g/mole= 100 g/moleCaO: 40 g/mole + 16 g/mole= 56 g/moleCO₂: 12 g/mole + 2*16 g/mole= 44 g/moleThen, by stoichiometry of the reaction, the following mass amounts of the compounds participating in the reaction react and are produced:
CaCO₃: 1 mole* 100 g/mole= 100 gCaO: 1 mole* 56 g/mole= 56 gCO₂: 1 mole* 44 g/mole= 44 gYou can then apply the following rule of three: if by stoichiometry of the reaction 100 grams of calcium carbonate CaCO₃ produce 56 grams of calcium oxide CaO, 25 grams of CaCO₃ how much mass of CaO will it produce?
\(mass of calcium oxide=\frac{25 grams of CaCO_{3} *56 grams of CaO}{100 grams of CaCO_{3} }\)
mass of calcium oxide= 14 grams
14 grams of calcium oxide would be produced by thermal decomposition of 25 grams of calcium carbonate.
Calcium bromide is the product of calcium and bromide ions. what is the formula for calcium bromide?
a. cacl2
b. CaBr2
c. CaO
d. Ca(OH)2
Answers
The combination of calcium with bromide ions results in calcium bromide. The chemical formula with calcium bromide is CaO.
What is produced when calcium bromide is used?Bromine and calcium oxide are created when calcium bromide is heated vigorously in air. 2 CaO + 2 Br from 2 CaBr2 + O2. The bromide is converted to bromine in this process by the oxygen.
Which calcium ion corresponds to the bromine ion?What about the substance created when calcium and bromine combine? Calcium loses two electrons in order to really be isoelectronic with argon, becoming the 2+ ion Ca2+. The 1-ion, Br-, is formed by bromine. Calcium bromide has the chemical formula CaBr2.
To know more about calcium bromide visit:
https://brainly.com/question/15351499
#SPJ4
If the solubility of a substance in water is 360 g/L and the molar mass of the substance is 58.5 g/mol. What is the Molarity of the saturated solution? Explain in your own words in complete sentences.
Answers
This means that for every liter of water, there is 6.15 moles of the substance dissolved in it.
What is substance?Substance is a concept that refers to a physical material or thing that has mass and occupies space. It is a fundamental concept of physics that applies to all physical and visible things in the universe. In philosophy, substance is a primary category of ontology that refers to the physical or material existence of things.
The molarity of the saturated solution can be calculated by dividing the solubility (360 g/L) by the molar mass of the substance (58.5 g/mol).
The molarity of the saturated solution is thus 6.15 mol/L.
This means that for every liter of water, there is 6.15 moles of the substance dissolved in it.
To learn more about substance
https://brainly.com/question/26487468
#SPJ1
Explain why the kidneys are important to our survival
1. They filter waste from the blood
2. They remove urine from the body
3. They increase the sugar level in the blood
4. They make new blood for the body
Answers
The kidneys are important to our survival because they play a critical role in maintaining the balance of fluids and electrolytes in the body, as well as removing waste and toxins from the blood. Options 1 and 2 are correct.
The kidneys filter waste from the blood, such as excess water, salts, urea, and other waste products. They remove urine from the body, which helps to maintain the proper balance of fluids and electrolytes in the body. The kidneys do not increase the sugar level in the blood, but they do play a role in regulating blood sugar levels by producing hormones such as renin and erythropoietin.
The kidneys do not make new blood for the body, but they do produce a hormone called erythropoietin that stimulates the production of red blood cells in the bone marrow. The kidneys are essential to our survival because they help maintain the proper balance of fluids and electrolytes, remove waste and toxins from the blood, and play a role in regulating blood pressure and red blood cell production. Options 1 and 2 are correct.
To know more about the Kidneys, here
https://brainly.com/question/11008782
#SPJ4
who discovered silver and how?
Answers
Answer:
Silver was discovered in Greece
Answer:
En 1516 Juan Díaz de Solís descubrió en Sudamérica el mar Dulce que posteriormente Sebastián Caboto denominó Río de la Plata, creyendo que allí abundaba el precioso metal, y de donde tomará el nombre la Argentina.
Explanation:
How can nuclear energy be used in medicine
Answers
The plantlets are uniform.
Answers
Answer:
yes they are < 3
Explanation:
3 Apply Many scientific words, such as energy, also have everyday meanings. Use context clues to write your own definition for each meaning of the word energy.
Example Sentence
I am tired today and don't have much energy.
Energy:
____________
Example Sentence
Make sure to turn off the lights when you're done to save energy.
Answers
Answer:
Explanation:
3 Apply Many scientific words, such as energy, also have everyday meanings. Use context clues to write your own definition for each meaning of the word energy.
How much heat (in kJ ) is required to warm 12. 0 g of ice, initially at -11. 0 ∘C , to steam at 109. 0 ∘C ? The heat capacity of ice is 2. 09 J/g⋅∘C and that of steam is 2. 01 J/g⋅∘C
Answers
36.607 kJ of heat are needed to warm 12.0 g of ice.
For grade 11, how do you calculate enthalpy change?By deducting the total enthalpies of all the reactants from the total enthalpies of the products, the reaction enthalpy is determined. According to mathematics, tH is equal to the sum of the enthalpies of the reactants and the product.
The following formula can be used:
q1 = m ×Cice × ΔT1
When we change the values, we obtain:
q1 = 12.0 g ×2.09 J/g⋅∘C × 11.0°C
q1 = 273.48 J or 0.27348 kJ
We must apply the following formula to melt the ice at 0 degrees Celsius:
q2 = m * ΔHfus
where m is the mass of the ice (12.0 g), q2 is the necessary heat, and Hfus is the heat of fusion for water (334 J/g).
When we change the values, we obtain:
q2 = 12.0 g × 334 J/g
q2 = 4008 J or 4.008 kJ
We can use the formula below to warm the water from 0°C to 100°C:
q3 = m * Cwater * ΔT2
When we change the values, we obtain:
q3 = 12.0 g × 4.18 J/g⋅∘C * 100°C
q3 = 5025.6 J or 5.0256 kJ.
The following formula must be used to bring the water to a boil at 100°C:
q4 = m * ΔHvap
When we change the values, we obtain:
q4 = 12.0 g × 2257 J/g
q4 = 27,084 J or 27.084 kJ.
Lastly, we may apply the following formula to raise the steam's temperature from 100°C to 109°C:
q5 = m × Csteam × ΔT3
When we change the values, we obtain:
q5 = 12.0 g × 2.01 J/g⋅∘C × 9°C
q5 = 216.36 J or 0.21636 kJ
The total heat required is the sum of all these values:
Q1 + Q2 + Q3 + Q4 + Q5 = Qtotal
Qtotal = 0.21636 kJ +4.008 kJ +5.0256 kJ +27.084 kJ.
qtotal = 36.607 kJ
To know more about heat visit:-
https://brainly.com/question/14072357
#SPJ4
Energy levels of electrons (n) indicates the distance of the energy level from the _______ values of n are positive integers n=1 is closest to the nucleus, and________ in energy
Answers
Energy levels of electrons (n) indicates the distance of the energy level from the nucleus values of n are positive integers n=1 is closest to the nucleus, and lower in energy
Any system which is in bounded state is consider to have negative potential energy and negative total energy.
The energy of the electrons rotating in the energy level is given as,
\(E_{n} = - 13.6/n^{2} eV\)
\(E_{n}\) is energy of the n level.n is the principle quantum number.So, on increasing value principle quantum number (n) the energy of the level is become higher.
The first energy level can contain to 2 electrons , the second energy levels can contain 8 electrons and third energy levels can contain 18 electrons and fourth energy levels can contain 32 electrons
learn about energy levels
https://brainly.com/question/17396431
#SPJ4
an ion of charged +4 has 21 electrons remaining in its atomic structure. what is the nunber of neutrons if it has a mass number of 55? A. 21 B. 25 C. 24 D. 30
Answers
An ion of charged +4 has 21 electrons remaining in its atomic structure. 30 is the number of neutrons if it has a mass number of 55. The correct option is option D.
Every atom's nucleus is made up of neutrons and protons, with the exception of common hydrogen, which nucleus only contains one proton. Neutrons are neutral subatomic particles. It is one of the three fundamental particles that make up atoms, the fundamental units of all matter & chemistry, together with protons and electrons.
The neutron is electrically neutral and has a rest mass of 1.67492749804 1027 kg, which is somewhat higher than the proton's but 1,838.68 times higher than the electron's.
atomic number = 21 + 4 = 25
mass number = 55
number of neutron = mass number- atomic number
= 55 -25
= 30
Therefore, the correct option is option D.
To know more about neutron, here:
https://brainly.com/question/28992636
#SPJ1
question 1 a spreadsheet cell contains the coldest temperature ever recorded in new zealand: -22 °celsius. what function will display that temperature in fahrenheit?
Answers
When the temperature conversion function =CONVERT(-22, "C", "F") is applied, a reading of -22 °C in Fahrenheit is displayed. On a variety of scales, including the Fahrenheit and Celsius systems, temperature is a unit that is used to denote hotness or coolness.
Heat energy will logically go from a hotter (body with a higher temperature) to a colder (body with a lower temperature) according to temperature (one at a lower temperature).
A temperature is a measurement used to express how hot or cold something is. It demonstrates how heat energy naturally flows from a hotter body to a cooler body and can be expressed in terms of any number of arbitrary scales (one at a lower temperature).
A match is burning at a far greater temperature than an iceberg, yet an iceberg has a significantly higher total heat energy than a match. Temperature is not the same as the energy of a thermodynamic system.
The temperature, along with pressure, density, and other similar properties, is referred to as an intense property as opposed to extensive characteristics like mass or volume—one that is independent of the quantity of stuff being addressed.
To know more about temperature:
brainly.com/question/23411503
#SPJ4
Determine whether each statement describes a solution of a strong electrolyte, weak electrolyte, or non-electrolyte.
Answers
Please find the terms with their correct description in the explanation section.
In an aqueous solution, an electrolyte is a material that separates or ionizes into cations (positively charged ions) and anions (negatively charged ions). Depending on how well it ionizes and how much conductivity it has, an electrolyte can be either strong or weak. A non-electrolyte, however, does not conduct electricity or ionize in a solution.
Based on this;
1. Weak electrolyte: Has a medium level of conductivity i.e. partially conducts electricity
2. Strong electrolyte: Contains a complete solute
3. Non-electrolyte: Has little or no conductivity i.e. cannot conduct electricity because it doesn't dissociate into ions.
4. Strong electrolyte: Has the highest conductivity i.e. conducts electricity very well.
5. Strong electrolyte: Contains a completely dissociated solute i.e. the solute of the electrolyte separates into anions and cations completely.
6. Weak electrolyte: Contains a partially dissociated solute i.e. the ions of the solute do not ionize completely in the solution.
To learn more about electrolyte visit:https://brainly.com/question/28699046
#SPJ1
What mass of uranium contains the same number of atoms as 25. 0 g of potassium (can you the work please)
Answers
The number of atoms in a given mass of a substance is determined by its atomic mass, which is the average mass of an atom of that element relative to 1/12 the mass of a carbon-12 atom.
Potassium has an atomic mass of 39.098 g/mol, so 25.0 g of potassium contains 25.0 g / 39.098 g/mol = 639.0 mol of potassium atoms.
Uranium has an atomic mass of 238.0 g/mol, so the mass of uranium that contains the same number of atoms as 25.0 g of potassium is 639.0 mol x 238.0 g/mol = 152,062 g of uranium.
What is the most common isotope of uranium found in nature?The most common isotope of uranium found in nature is uranium-238. It makes up over 99% of naturally occurring uranium and has a half-life of over 4 billion years. It is primarily used as a fuel in nuclear reactors.
To know more about Atomic Mass visit: https://brainly.com/question/5661976
#SPJ4
a single electron in an orbital has quantum numbers n = 3, ℓ = 0, mℓ = 0, ms =-½. what are the quantum numbers for the next electron added to this orbital?
Answers
The quantum numbers for the next electron added to this orbital are n = 3, ℓ = 0, mℓ = 0, and ms = +½.
The quantum numbers for an electron in an orbital describe its energy level, angular momentum, magnetic moment, and spin. Based on the given quantum numbers for the first electron, we can determine the possible quantum numbers for the next electron added to this orbital.
The Pauli exclusion principle states that no two electrons in an atom can have the same set of four quantum numbers, so the quantum numbers for the second electron must be different from the first electron.
We know that the first electron has the following quantum numbers:
Principal quantum number (n) = 3
Azimuthal quantum number (ℓ) = 0
Magnetic quantum number (mℓ) = 0
Spin quantum number (ms) = -½
Since the azimuthal quantum number (ℓ) is 0, the first electron is in an s orbital. An s orbital can hold a maximum of 2 electrons, and when two electrons are in the same orbital, they must have opposite spins.
Therefore, the quantum numbers for the second electron added to this orbital must be:
Principal quantum number (n) = 3
Azimuthal quantum number (ℓ) = 0
Magnetic quantum number (mℓ) = 0 (since the s orbital has only one orientation)
Spin quantum number (ms) = +½ (since it must have the opposite spin to the first electron, which has ms = -½).
For such more questions on Quantum number
https://brainly.com/question/25786066
#SPJ4
lewis structure of isopropanol
Answers
The isopropanol molecule comprises a total of 11 bond three non-H bonds, one hydroxyl group, and one secondary alcohol are present in the lewis structure of isopropanol.
The most popular and commonly used disinfectant in pharmaceutics, hospitals, cleanrooms, and electronics or medical device manufacture is isopropyl alcohol (2-propanol), sometimes known as isopropanol or IPA. The arrangement of atoms and the chemical bonds that hold them together make up a molecule's chemical structure. Isopropyl alcohol quickly kills bacteria, fungi, and viruses, especially in concentrations between 60% and 90% alcohol and 10–40% filtered water. Alcohol concentrations below 50% are no longer very useful for disinfection. An empty or full bonding, antibonding, or lone pair orbital can function as a donor, as can a filled bonding or lone pair orbital.
To learn more about lewis structure click here https://brainly.com/question/20300458
#SPJ4
How many molecules are in 48.0 grams of NaOH?
Answers
Hope this helped :)
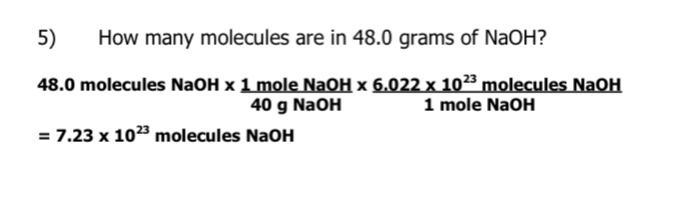
What is the salt called when it is mixed with water?
Answers
Answer:
NaCI (salt) in water
Explanation:
After the salt compounds are pulled away apart the sodium chloride atoms are surrounded by water molecules. Once this happens we know that the salt is dissolved resulting in a homogeneous solution!
HOPE THIS HELPED!
Answer:
NaCI (salt) in water
Explanation:
took the quiz
Evidence for past events in Earth's ancient history are provided by:
a
time lines
b
history books
c
the biodiversity of North America
d
rocks and the fossils within them
Answers
calculate how many kcals would be in a meal that contains 19 grams of fat, 39 grams of carbohydrate, and 16 grams of protein. 391 220 171 433
Answers
220 Calories would be in a meal that contains 19 grams of fat, 39 grams of carbohydrate, and 16 grams of protein.
calories are units of measurement for the amount of calories in a beverage or food. When we take in more calories than we burn, our carcasses store the excess as fat. If this persists, we may gain weight over time. To maintain a healthy weight, an average man requires approximately 2,500kcal (10,500kJ) per day. Calories are the units of energy is released by your body whenever it digests as well as absorbs food. The higher the calorie content of a food, the more power it can supply to your body. When you take in more calories than you require, your body will store the surplus calories as fat.
Learn more about Calories here:
https://brainly.com/question/22374134
#SPJ4
Which radioactive isotope is used in geological dating? (1) U-238, (2) I-131, (3) Co-60, (4) Tc-99.
Answers
The radioactive isotope used in geological dating is U-238. Option 1 is correct.
U-238 (uranium-238) is a radioactive isotope commonly used in geological dating. It has a half-life of about 4.5 billion years, which means that it takes that amount of time for half of the original amount of U-238 in a rock to decay into other elements, such as lead. By measuring the ratio of U-238 to lead in a rock sample, scientists can estimate how long it has been since the rock formed.
I-131 (iodine-131) is a radioactive isotope used in medical treatments and diagnostic procedures, Co-60 (cobalt-60) is used in radiation therapy for cancer treatment, and Tc-99 (technetium-99) is used in medical imaging procedures. These isotopes have different half-lives and decay processes and are not used for geological dating.
To know more about the radioactive isotope, here
brainly.com/question/1907960
#SPJ4
what makes a compound a pure substance
Answers
Answer:
Compounds contain more than one type of material. Yet both compounds and elements are considered pure substances.
Pure compounds are created when elements combine permanently, forming one substance
Extra:
So, a mixture can be separated into its original components, while a pure compound cannot.
how many bonds can sodium form
Answers
it exists as atom or ion but not as sodium molecule. So there is no any sodium sodium bonding between two sodium atoms. Sodium combines with other element or group to form compounds of sodium like sodium chloride ,sodium carbonate ,sodium sulphate etc. Sodium has symbol Na and its atomic no. is 11 .
Answer:
\(\boxed{One\: Ionic\: Bond}\)
Explanation:
For example, sodium, which is a metal, and chloride, a nonmetal, form an ionic bond to make NaCl.
Hope it helps!<3
How could you modify this simulation to demonstrate that different isotopes have different half lives
Answers
The simulation can be modified to demonstrate different isotopes have different half lives by \(-\frac{d[A]}{dt} = k[A]^n\)
Partial life is a partial life because it's defined this way. stay lower than that, and the decay will take out lower than partial tittles; stay more, and it'll take out further than half.
The partial life is used as a accessible measure because a sample of isotopes decays exponentially with time. Each snippet has the same chance of decaying in a fixed time interval and this leads to an exponential decay. All tittles bear the same way and continue to decay until all have been changed, whether this takes glories or seconds.
You can just as fluently define a" one- third" life, a" quarter- life". Radioactive isotopes decay exponentially; half- life is just accessible measure that captures the kinetics of the decay.
Learn more about Isotopes lives:
https://brainly.com/question/16387602
#SPJ4
Which of the following happens to a molecule of an object when the object is cooled?
Answers
Answer: It loses kinetic energy.
What is the stoichiometric ratio between the 20 mg band reactant and the product hydrogen gas in the reaction used to determine the molar volume of the gas?
Answers
The stoichiometric ratio between the 20 mg band reactant and the Product hydrogen gas is 1:1/2, simply 2:1. This means that 2 atoms of hydrogen are produced for every 1 atom of zinc consumed in the reaction.
What is Stoichiometric Coefficient?
A stoichiometric coefficient is a number that appears in front of a chemical species in a balanced chemical equation, representing the number of moles of that species that react or are produced in the reaction. These coefficients are used to ensure that the law of conservation of mass is obeyed in a chemical reaction, and that the same number and types of atoms are present on both sides of the equation.
The reaction used to determine the molar volume of a gas is typically the reaction between a metal and an acid to produce hydrogen gas, which is collected and measured. The stoichiometric ratio between the reactants and products in this reaction is:
metal + acid → salt + hydrogen gas
The balanced chemical equation for the reaction between zinc metal and hydrochloric acid is:
Zn + 2 HCl → ZnCl2 + H2
From this equation, we can see that 1 mole of zinc reacts with 2 moles of hydrochloric acid to produce 1 mole of hydrogen gas. The molar mass of zinc is 65.38 g/mol, which means that 1 mole of zinc has a mass of 65.38 g. Therefore, 20 mg (0.02 g) of zinc is equivalent to:
0.02 g / 65.38 g/mol = 3.06 x 10^-4 moles of zinc
According to the stoichiometry of the reaction, 1 mole of zinc reacts with 1/2 mole of hydrogen gas. Therefore, the amount of hydrogen gas produced from 3.06 x 10^-4 moles of zinc is:
1/2 x 3.06 x 10^-4 moles = 1.53 x 10^-4 moles of hydrogen gas
So the stoichiometric ratio between the 20 mg band reactant and the product hydrogen gas is 1:1/2, or simply 2:1. This means that 2 atoms of hydrogen are produced for every 1 atom of zinc consumed in the reaction.
Learn more about Stoichiometric Coefficient from given link
https://brainly.com/question/6666875
#SPJ4
2. Give an example of an atom found in the human body (both its common name and the atomic symbol). 3. What is an ion? 4. Give an example of an ion found in the human body (both its common name and its symbol). 5. What are covalent bonds?
Answers
They are the most common type of bond in organic molecules. These bonds are important in biological systems, as they hold the molecules of life together. They are typically strong and difficult to break, making them important in the structure of large molecules such as proteins and DNA.
2. An atom found in the human body (both its common name and the atomic symbol): An example of an atom found in the human body is Carbon.
Its atomic symbol is C.3. Ion:
An ion is an atom or molecule that has an unequal number of protons and electrons. It can be negatively charged (anion) or positively charged (cation).4. An ion found in the human body (both its common name and its symbol): An example of an ion found in the human body is Sodium. Its symbol is Na, and it has a positive charge (cation).5. Covalent bonds: Covalent bonds are chemical bonds that are formed when two atoms share a pair of electrons. They are the most common type of bond in organic molecules. These bonds are important in biological systems, as they hold the molecules of life together. They are typically strong and difficult to break, making them important in the structure of large molecules such as proteins and DNA.
To know more about molecules visit:
https://brainly.com/question/32298217
#SPJ11
Glass all starts with ordinary ___________, which is made of a combination of silicon and oxygen.
Answers
Glass all starts with ordinary sand, which is made of a combination of silicon and oxygen. Glass is a solid material that is typically made by heating a mixture of various raw materials.
Glass is made by heating a mixture of materials including sand, soda (sodium carbonate), and limestone (calcium carbonate), to a high temperature until it melts and then allowing it to cool and solidify. The main ingredient in most types of glass is silica, which is derived from sand. Sand is composed of silicon dioxide (SiO₂) , which is a compound made up of silicon (Si) and oxygen (O) atoms. Silicon is a chemical element that is abundant in the Earth's crust and is a key component of many minerals, including quartz, which is a common type of sand.
Learn more about Glass here:
https://brainly.com/question/29775274
#SPJ11
How do you think the arrangement of particles in different states of matter is related to the properties of those states of matter?
Answers
jenial pues muy bien con eso propuesta .........aaaaaaaaaaaaa
Answer:
Particles in a: gas are well separated with no regular arrangement. liquid are close together with no regular arrangement. solid are tightly packed, usually in a regular pattern.