Answers
Answer:
Hydrogen.
Explanation:
You've probably seen "\(H_{2}0\)" which is the formula for water. It means that there's 2 hydrogen atoms, and one oxygen atom, in one molecule of water.
Hope this helps! Feel free to mark me Brainliest if you feel this helped. :)

Answer:
the answer is H. (hydrogen)
Related Questions
Which of the following reactions would result in decreased entropy?
O A. H20(g) → H20(1)
O B. CO2(s) → CO2(9)
O C. 203(9) ► 302(9)
O D. N2204(9) ► 2NO39)
Answers
Answer:
A
Explanation:
A P E X
A maple tree could be studied in many fields of science. What aspects of a maple tree might be studied in chemistry?
Answers
Answer:
Chemical reactions, kinetics, organic chemistry
Explanation:
You might study the chemical reaction, learn about the differences between products and reactants, about delta H and exothermic and endothermic reactions. You may also study Kinetics by studying the rates of reactions with certain chemicals in a maple's enzymatic processes.
Another thing that you might learn about is organic chemistry. The glucose molecules, carbohydrates, lipids, nucleic acids, all have a structure based on the Carbon atom. You can learn about the specific structures of some chemicals that are involved in photosynthesis and simple hydrocarbons that are involved in photosynthetic/bio-synthetic pathways.
There's probably a lot more - but these are the most basic things I could think of.
According to the Arrhenius equation, changing which factors will affect the
rate constant?
OA. The constant A and the temperature
B. Temperature and activation energy
C. Temperature and the ideal gas constant
D. The activation energy and the constant A
SUBMIT
Answers
The correct answer is B: Temperature and activation energy are the factors that affect the rate constant according to the Arrhenius equation.
According to the Arrhenius equation, which describes the relationship between the rate constant (k) of a chemical reaction and temperature, changing the factors of temperature and activation energy will affect the rate constant.
The Arrhenius equation is given by:
k = A * e^(-Ea/RT),
where k is the rate constant, A is the pre-exponential factor or the frequency factor, Ea is the activation energy, R is the ideal gas constant, T is the temperature in Kelvin, and e is the base of the natural logarithm.
From the equation, it is evident that the rate constant is directly influenced by temperature and activation energy. Increasing the temperature results in a higher rate constant, as the exponential term in the equation becomes larger. This is because higher temperatures provide more kinetic energy to the reacting molecules, leading to more frequent successful collisions and increased reaction rates.
Similarly, the activation energy affects the rate constant. A higher activation energy results in a lower rate constant, as the exponential term becomes smaller. Activation energy represents the energy barrier that reactant molecules must overcome to form products. A higher activation energy implies a slower reaction rate. option(B)
For such more questions on Temperature
https://brainly.com/question/30668924
#SPJ8
Scientists currently use radioactive isotopes in various field. Some radioactive isotopes are used to _____.
•power iasers
•develop new antibiotics
•clone organisms
•date ancient bones
Answers
\(\huge{\textbf{\textsf{{\color{pink}{An}}{\red{sw}}{\orange{er}} {\color{yellow}{:}}}}}\)
ur answer is:-
date ancient bones
Scientists currently use radioactive isotopes in various field. Some radioactive isotopes are used to date ancient bones.
What are isotopes?Isotopes are defined as substances having same number of protons but different number of neutrons.Number of protons is characteristic for determining position of elements in the periodic table.
Since,all isotopes have the same number of protons and hence have same position.They have similar chemical properties as they have same number of electrons.
They find applications in the field of nuclear medicine and oil and gas research . There are 2 types of isotopes : stable and unstable
Unstable isotopes are radioactive and are called as radioisotopes.Some of these isotopes are man -made and hence also called as artificial isotopes.Every element has an isotope which is either man-made or natural .
Many properties of isotopes depend on mass which is measured in atomic mass unit. The difference in actual mass and mass number is called mass defect.
Learn more about isotopes,here:
https://brainly.com/question/21536220
#SPJ6
Help pls i really need help

Answers
Answer: I think it's 1
Explanation: Potential energy means stored energy . In Position 1 it's not moving the energy is being stored. Hope that helps.
How many Tootsie Rolls can you get for 14 Bit o' honey?
Answers
Help pls 50 points and brainliest.
Matter is made up of heat energy and______energy.
1. chemical
2. light
Answers
Answer:
Matter is Made up of Chemical Energy..
Answer:
\(\huge\boxed{\sf Chemical}\)
Explanation:
Matter is made up of atoms. Atoms contain chemical bonds which hold up the atoms together. These chemical bonds store chemical energy in themselves. Hence Matter is made up of chemical energy and heat energy.
Light energy have no relation with matter. Remember, Light is not a matter and matter is not light.
\(\rule[225]{225}{2}\)
Hope this helped!
~AH1807Maya adds six grams of sugar to water bottle containing 14 g of warm water what is the total weight of the sugar and water
Answers
Answer:
20g
Explanation:
14g+6g=20g
Question 2 of 10
What is the percent yield of a reaction?
The amount of product obtained x 100
amount possible
B. The amount of product actually obtained in a reaction
C. The amount of product that is possible from a reaction
D. The difference between measured and calculated amounts
A.
Answers
Answer:
c
Explanation:
NCl3 + 3H20 - NH3 + 3HCIO
How many grams of ammonia can be produced from 1.33 grams of nitrogen trichloride?
Answers
Answer:
0.189 g
Explanation:
Step 1: Write the balanced equation
NCl₃ + 3 H₂O ⇒ NH₃ + 3 HCIO
Step 2: Calculate the moles corresponding to 1.33 g of NCl₃
The molar mass of NCl₃ is 120.36 g/mol.
1.33 g × 1 mol/120.36 g = 0.0111 mol
Step 3: Calculate the moles of NH₃ produced from 0.0111 moles of NCl₃
The molar ratio of NCl₃ to NH₃ is 1:1. The moles of NH₃ produced are 1/1 × 0.0111 mol = 0.0111 mol.
Step 4: Calculate the mass corresponding to 0.0111 moles of NH₃
The molar mass of NH₃ is 17.03 g/mol.
0.0111 mol × 17.03 g/mol = 0.189 g
Which seasons in Atlanta GA have worst AQI
Answers
In Atlanta, GA, certain seasons are associated with poorer air quality due to various factors such as weather conditions, human activities, and geographical location.
Typically, the seasons with the worst AQI in Atlanta, GA, are summer and early fall. This is primarily due to the combination of high temperatures, stagnant air masses, and increased pollution from various sources.
During the summer months, Atlanta experiences hot and humid weather, which can contribute to the formation of ground-level ozone. Ozone is a harmful pollutant that is created when pollutants from vehicles, power plants, and industrial activities react with sunlight and heat. High levels of ozone can cause respiratory issues and other health problems.
In addition to ozone, Atlanta also experiences increased levels of particulate matter (PM) during the summer and early fall. PM refers to tiny particles suspended in the air, which can come from sources such as vehicle exhaust, industrial emissions, and wildfires.
These particles can be inhaled into the lungs and can have detrimental effects on respiratory health.
It's important to note that air quality can vary from year to year and is influenced by various factors. Local regulations, weather patterns, and changes in pollutant emissions can all impact the AQI during different seasons.
Monitoring air quality reports and taking necessary precautions such as reducing outdoor activities during times of poor air quality can help individuals stay informed and protect their health.
For more such question on air quality visit:
https://brainly.com/question/21173066
#SPJ8
heat transfer through any intermediate fluid is known as?
Answers
Answer:
convection
Explanation:
Answer: At the beach, differences in temperatures create wind which transfers heat between the land and the water. ... Heat transfer through any intermediate fluid is known as
(A.) Condensation.
What is the concentration of silver ions where silver iodide, Agl, is in a solution of hydroiodic
acid, HI, that has a pH of 3.55? Ksp = 8.51x10-17
Answers
Answer:
\([Ag^+]=2.82x10^{-4}M\)
Explanation:
Hello there!
In this case, for the ionization of silver iodide we have:
\(AgI(s)\rightleftharpoons Ag^+(aq)+I^-(aq)\\\\Ksp=[Ag^+][I^-]\)
Now, since we have the effect of iodide ions from the HI, it is possible to compute that concentration as that of the hydrogen ions equals that of the iodide ones:
\([I^-]=[H^+]=10^{-3.55}=2.82x10^{-4}M\)
Now, we can set up the equilibrium expression as shown below:
\(Ksp=8.51x10^{-17}=(x)(x+2.82x10^{-4})\)
Thus, by solving for x which stands for the concentration of both silver and iodide ions at equilibrium, we have:
\(x=[Ag^+]=2.82x10^{-4}M\)
Best regards!
What is the mole ratio of ammonia (with a pKb of 4.75) to ammonium chloride in a buffer with a pH of 9.15
Answers
The Henderson-Hasselbalch equation can be used to calculate the mole ratio of ammonia to ammonium chloride pH is equal to pKb plus \(log(NH3/NH4Cl).\)
The equation can be changed to answer the question: What is the mole ratio of ammonia?Figure 1 depicts the chemical equation for producing ammonia and demonstrates that the mole ratio of ammonia to nitrogen gas is \(2:1\). As seen in the chemical reaction, one mole of nitrogen gas results in the production of two moles of ammonia.
How is the mole ratio determined?By dividing the total number of moles by the smallest number of moles, you may determine the ratio or the number of moles of each element.
To know more about ammonia visit:-
brainly.com/question/15409518
#SPJ9
What is the formula for the molecule above? (Periodic table squares are provided below,
if you want them.)
A. H2
B. H2O
C.O2
D. H

Answers
Answer:
I believe H2.
Explanation:
It's H2 because there are two electrons on there being share by 2 atoms.
Since Hydrogen can only get a maximum of 2 electrons and no other atoms are present, that is why it's H2.
Could someone tell what element your
specific atom is based on your model?
Explain.
help! plz
Answers
Answer:
yes
Explanation:
the number of protons in the nucleus of an atom determines the type element: the number of protons in an element is the atomic number on the periodic table (number at the top)
Each atom has characteristic number of protons present in it's nucleus which is unique for that element.
What is an element?An element is defined as a substance which cannot be broken down further into any other substance. Each element is made up of its own type of atom. Due to this reason all elements are different from one another.
Elements can be classified as metals and non-metals. Metals are shiny and conduct electricity and are all solids at room temperature except mercury. Non-metals do not conduct electricity and are mostly gases at room temperature except carbon and sulfur.
The number of protons in the nucleus is the defining property of an element and is related to the atomic number.All atoms with same atomic number are atoms of same element.
Learn more about element,here:
https://brainly.com/question/24407115
#SPJ2
Select the structure that corresponds
to the name:
N, N-diethylpropanamide

Answers
Answer:
B
Explanation:
Astronauts must be protected from extreme heat while
reentering the Earth's atmosphere. Scientists can use the
engineering design process to help make reentry safer.
You have defined the problem as a need for heat shields to be stronger. What
should be your next step in using the engineering design process to solve the
problem?
O A. Communicate your solution to your team members.
O B. Update your initial design for the heat shield.
O C. Identify the criteria and constraints of the project.
O D. Build a prototype of a possible new type of heat shield.
Answers
The next engineering step to be taken is to update your initial design for the heat shield.
What is a heat sheild?The term heat sheild refers to a device that can be used to shield an from astronaut extreme heat especially as they re-enter the earth's atmosphere.
After you have identified the problem, the next engineering step to be taken is to update your initial design for the heat shield.
Learn more about heat sheild:https://brainly.com/question/21504467?
#SPJ2
A 0.563 M solution of the salt NaA has a pH of 11.56. Calculate the Ka value for the acid HA. Record your answer in scientific notation to 3 sig figs.
Answers
Answer:
\(\displaystyle K_a = 4.24\times 10^{-10}\)
Explanation:
Write the base reaction of NaA with water:
\(\displaystyle \text{A}^-_\text{(aq)}+\text{H$_2$O}_\text{($\ell$)}\rightleftharpoons \text{HA}_\text{(aq)} + \text{OH}^-_\text{(aq)}\)
Hence, the equilibrium constant expression for the reaction is:
\(\displaystyle K_b = \frac{[\text{OH}^-][\text{HA}]}{[\text{A}^-]}\)
Thus, to find Ka, we can find Kb and use the fact that Ka × Kb = Kw.
From the reaction and initial concentration of NaA, create an ICE chart:
\(\begin{tabular}{llllll} & A^- &\text{H$_2$O} & \rightleftharpoons & HA & OH^- \\I & 0.563 M & \---- & & 0 M & 0 M \\C & -\text{ $ x$} & \---- & & +\text{ $x$ M} & + \text{$x$ M} \\E & \text{(0.563 - $x$) M} & \---- & & \text{$x$ M} & \text{$x$ M} \end{tabular}\)
Find [OH⁻] from the given pH:
\(\displaystyle \begin{aligned} \text{pH} +\text{pOH} & = 14.00 \\ \\ \text{pOH} & = 14.00 - \text{pH} \\ \\ & = 14.00 - (11.56) \\ \\ & = 2.44 \\ \\ -\log[\text{OH}^-] & = 2.44 \\ \\ [\text{OH}^-] &= 10^{-2.44} \\ \\ & =0.00363 \text{ M}= 3.63\times 10^{-3} \text{ M} = x\text{ M}\end{aligned}\)
Solve for all species concentrations at equilibrium from the found x value:
\(\displaystyle [\text{HA}] = [\text{OH}^-] = 3.63\times 10^{-3} \text{ M}\)
And:
\(\displaystyle \begin{aligned} \ [\text{A}^-] & = 0.563 - 3.63\times 10^{-3} \text{ M}\\ \\ & = 0.559\text{ M}\end{aligned}\)
Find Kb:
\(\displaystyle \begin{aligned} \displaystyle K_b &= \frac{[\text{OH}^-][\text{HA}]}{[\text{A}^-]} \\ \\ & = \frac{(3.63\times 10^{-3})(3.63\times 10^{-3})}{(0.559)}\\ \\ & = 2.36\times 10^{-5}\end{aligned}\)
Find Ka:
\(\displaystyle \begin{aligned} K_a\cdot K_b & = K_w \\ \\ K_a & = \frac{K_w}{K_b} \\ \\ & = \frac{(1.00 \times 10^{-14})}{(2.36\times 10^{-5})} \\ \\ &= 4.24\times 10^{-10} \end{aligned}\)
In conclusion:
\(\displaystyle K_a = 4.24\times 10^{-10}\)
A piece of a metal having a mass of 150.0000 grams is heated to 99.3 9C. When the hot metal is submerged in 50.000 grams of water originally at 21.3 oC, the final temperature of the water and metal is 48.4 pC. Determine the heat flow (q) of the water in Joules Record the answer to the correct number of significant figures. Show the numerical set-up(s) and include the units in the set-ups. a) Does the temperature of the water increase, decrease, or remain the same? b) Is the heat flow of the water positive, negative, or zero? c) Does the temperature of the metal increase, decrease, or remain the same? d) Is the heat flow of the metal positive, negative, or zero? If the heat gained by the water is the same heat that is lost by the metal, what is the heat flow of the 150.000 gram sample of metal, in Joules? Record your answer in the box to the correct number of significant figures. Determine the value of the specific heat of the 150.000 gram sample of metal, in J/g*oC. Record your answer, to the correct number of significant figures, in the box. Show the numerical set-up(s) and be sure to include the units in your set-ups.
Answers
Answer:
Explanation:
a ) The temperature of water increases from 21.3°C to 48.4°C .
b ) Heat flow of water ( q) is positive because there is rise in temperature .
c ) The temperature of metal decreases from 99.3⁰C to 48.4⁰C .
d ) Heat flow of metal is negative because there is fall in temperature .
e ) heat loss of metal = heat gain by water
heat gain by water ( q ) = 50 x 4.2 x ( 48.4 - 21.3 )
= 5691 J
q = 5690 J . ( 3 significant figures )
heat loss of metal = mass x specific heat x fall in temp = 5691
150 x specific heat x ( 99.3 - 48.4 ) = 5691
specific heat = 5691 / (150 x 50.9 )
= .745 J / g C ( three significant figures )
Calculate the number of grams of platinum in 0.00808 moles Pt.

Answers
Answer:
1.58 g Pt
Explanation:
one mole of platinum weighs 195.09 grams (195.09 g/mol).
to convert moles of platinum to grams, we simply multiply the given moles of platinum by 195.09 to convert it to grams.
0.00808 x 195.09 = 1.576 g
How many grams of NaCl

Answers
You would recover 36.525g of NaCl after evaporating all of the water.
How to find the how many grams of NaCl that would be recover when all water is evaporated off of this solution?To find the grams of NaCl that would be recovered after evaporating all the water, we can use the following formula:
mass = moles * molar mass
Where:
Moles = Molarity * Volume
Molarity = 0.250 M
Volume = 2500.0 mL = 2.5 L
Molar mass of NaCl = 58.44 g/mol
mass = 0.250 M * 2.5 L * 58.44 g/mol
mass = 36.525 g
Learn about evaporation here https://brainly.com/question/2013258
#SPJ1
HQ5.40
Homework Answered Due Today, 11:59 PM
The reaction 3H₂(g) + N₂(g) → 2NH3(g) has an enthalpy of reaction of -92.6 kJ/mol. If 1 g of hydrogen and 2 g of nitrogen are
reacted, how much heat is produced (kJ)?
Answers
The amount of heat energy produced when 1 g of hydrogen and 2 g of nitrogen are reacted, is -6.61 KJ
How do i determine the heat energy produced?First, we shall obtain the limiting reactant. Details below:
3H₂ + N₂ -> 2NH₃
Molar mass of N₂ = 28 g/molMass of N₂ from the balanced equation = 1 × 28 = 28 g Molar mass of H₂ = 2 g/molMass of H₂ from the balanced equation = 3 × 2 = 6 gFrom the balanced equation above,
28 g of N₂ reacted with 6 g of H₂
Therefore,
2 g of N₂ will react with = (2 × 6) / 28 = 0.43 g of H₂
We can see that only 0.43 g of H₂ is needed in the reaction.
Thus, the limiting reactant is N₂
Finally, we the amount of heat energy produced. Details below:
3H₂ + N₂ -> 2NH₃ ΔH = -92.6 KJ
Molar mass of N₂ = 28 g/molMass of N₂ from the balanced equation = 1 × 28 = 28 gFrom the balanced equation above,
When 28 grams of N₂ reacted, -92.6 KJ of heat energy were produced.
Therefore,
When 2 grams of N₂ will react to produce = (2 × -92.6) / 28 = -6.61 KJ
Thus the heat energy produced from the reaction is -6.61 KJ
Learn more about heat energy:
https://brainly.com/question/31429264
#SPJ1
At what state is bromine at 100 degree
Answers
Answer:
a liquid
Explanation:
61. Given the following information:
Ag2 CrO4(s)=2Agt (aq) + CrO4²- (aq)
Ag+ (aq) + e- Ag(s)
find the standard reduction potential at 25°C for the half-reaction
Ksp = 1 × 10-12
E = +0.799 V
Ag2 CrO4(s) + 2e¯ 2Ag(s) + CrO4²- (aq)
Answers
Q = Ksp = 1 × 10^(-12).
Substituting the values into the Nernst equation, we have:
0.799 V = E° - (RT/2F) * ln(1 × 10^(-12))
Now, solving for E°:
E° = 0.799 V + (RT/2F) * ln(1 × 10^(-12))
The value of R is the ideal gas constant, T is the temperature in Kelvin, and F is the Faraday constant.
To find the standard reduction potential at 25°C for the half-reaction Ag2CrO4(s) + 2e¯ → 2Ag(s) + CrO4²-(aq), we can use the Nernst equation, which relates the standard reduction potential (E°) to the equilibrium constant (K) and the reaction quotient (Q).
The Nernst equation is given as follows:
E = E° - (RT/nF) * ln(Q)
Given information:
Ksp = 1 × 10^(-12)
E = +0.799 V (standard reduction potential of Ag+ to Ag)
Since the reaction involves the dissolution of Ag2CrO4(s), the reaction quotient Q can be expressed as [Ag+]²/[CrO4²-].
Since the stoichiometry of the reaction is 2:1 for Ag2CrO4 to Ag+, we can say that [Ag+]² = Ksp.
Therefore, Q = Ksp = 1 × 10^(-12).
Substituting the values into the Nernst equation, we have:
0.799 V = E° - (RT/2F) * ln(1 × 10^(-12))
Now, solving for E°:
E° = 0.799 V + (RT/2F) * ln(1 × 10^(-12))
The value of R is the ideal gas constant, T is the temperature in Kelvin, and F is the Faraday constant.
Please note that without specific values for temperature (T) and the ideal gas constant (R), the exact standard reduction potential at 25°C cannot be determined.
For more question on temperature
https://brainly.com/question/4735135
#SPJ8
What is TRUE about expert witnesses?
A.
They offer personal and professional knowledge.
B.
They are not allowed to practice their testimony.
C.
They are required to link all evidence to the crime.
D.
They have all written a book about their area of expertise.
Answers
The true statement about is expert witnesses that they are required to link all evidence to the crime.
So, option C is correct.
Who are expert witnesses?An expert witness is described particularly in common law countries such as the United Kingdom, Australia, and the United States, is a person whose opinion by virtue of education, training, certification, skills or experience, is accepted by the judge as an expert.
Expert witnesses are required or expected to to link all evidence to the crime.
Learn more about expert witnesses at: https://brainly.com/question/4448063
#SPJ1
A chemist must prepare 900.0 mL of potassium hydroxide solution with a pH of 12.20 at 25°C.
He will do this in three steps:
• Fill a 900.0 mL volumetric flask about halfway with distilled water.
• Weigh out a small amount of solid potassium hydroxide and add it to the flask.
. Fill the flask to the mark with distilled water.
Calculate the mass of potassium hydroxide that the chemist must weigh out in the second step. Round your answer to 2 significant digits and put your answer in grams (g).
Answers
Answer:
0.80 g
Explanation:
First we calculate the required pOH from the given pH value:
pOH = 14 - pHpOH = 14 - 12.20 = 1.80Then we calculate the required concentration of OH⁻, using the pOH:
pOH = -log[OH⁻][OH⁻] = \(10^{-1.80}\) = 0.0158 MAs the concentration of OH⁻ species is the sames as the concentration of KOH, we need to prepare 900 mL of a 0.0158 M KOH solution:
We calculate how many KOH moles are required, using the concentration and volume:
Converting 900 mL ⇒ 900 / 1000 = 0.900 Lmoles = 0.0158 M * 0.900 L = 0.01422 molFinally we convert 0.01422 moles of KOH to grams, using its molar mass:
0.01422 mol * 56 g/mol = 0.80 g3 H2S + 2AuCl --> Au2S3 + 6 HCL is what type
of reaction
Answers
Answer:
Double Displacement (Metathesis)
Explanation:
Determine whether each statement is an example
of a physical change or a chemical change.
Wood is burned.
physical
chemical
0
Dry ice (solid carbon dioxide) vaporizes to form
carbon dioxide gas.
Ophysical
chemical
DONE ✔
Answers
Answer:
Burning of wood is chemical change
Dry ice into Carbon dioxide conversion is physical change.
Explanation:
Because when wood is burnt it cannot be recovered back by tthe ashes. So it is chemical change.
Dry ice conversion into Carbon dioxide is a physical change because the chemical composition remains same.
Answer:
wood = chemical
dry ice = physical
Explanation:
Options for the first box: HF, HLiF, LiF, LiOHoptions for the second box: hydrofluoric acid, lithium hydroxide, lithium oxide, water
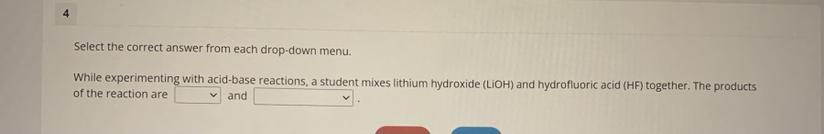
Answers
In acid base reactions, also known as neutralization reactions, the products are always a salt and water. The salt is formed with the cation of the base and the anion of the acid.
The anion of the acid is fluoride and the cation of the base is litium, it means that the salt formed is LiF.
For the second box, the answer is water.