Water can dissolve other substances. Which factor best contributes to this property of water?.
Answers
The property of polarity best contributes to the ability of water to dissolve other substances.
Water is a universal solvent because it can dissolve almost any substance due to its high polarity. This property of water makes it an excellent solvent for many substances. Water is an excellent solvent for polar and ionic substances because it has a high dielectric constant. Water has a slightly positive charge on one side and a slightly negative charge on the other due to its polar nature.
This dipole nature enables water molecules to attract ions and other polar molecules, effectively breaking them apart and dissolving them. This is because the positive side of the molecule attracts negatively charged ions, while the negative side of the molecule attracts positively charged ions. The polar nature of water makes it an excellent solvent for many substances. Water can dissolve a wide range of substances, including salts, sugars, and acids.
Learn more about polarity here:
https://brainly.com/question/1946554
#SPJ11
Related Questions
can you pls help me with this question

Answers
Answer:
probably c
Explanation:
Answer: I feel like the correct answer is choice C but I really don’t know how to explain it..
How many electrons does F^-1 have?
A. 9 electrons
B. 1 electron
C. 10 electrons
D. 8 electrons
Answers
Answer:
Florine has 9 atomic number so it has 9 electrons. So, add 1 to the number of electrons it has. 9 + 1 = 10. F-
Explanation:
Answer:
9 electrons
Explanation:
What is the rate of a reaction if the value of kis 3 and [A] and [B] are each 2
M?
Rate = K[A]²[B]
OA. 12 (mol/L)/s
OB. 24 (mol/L)/s
OC. 2 (mol/L)/s
о D. 36 (mol/L)/s
Answers
The rate of reaction of the value of KIS 3 and [A] and [B] are each 2M is: 24 (mol/L)/s (Option B)
What is rate of reaction?
In chemistry, the rate of reaction describes how quickly a chemical reaction develops.
It is frequently described in terms of either the concentration of a reactant that is spent in a unit of time or the concentration of a product that is generated in a unit of time (amount per unit volume).
What is the calculation that supports that above answer?
Recall that the rate equation is:
Rate = K[A]²[B]
Hence:
Concentration of A = 2M
Concentration of B = 2M
K = 3
Taking the values and substituting them, we have:
Rate = 3 (mol/L)⁻³/s×[2]² ×[2]
Rate = 24 (mol/L)⁻³/s
The rate of reaction can be used as a valuable diagnostic tool. We may devise strategies to increase production by learning how quickly things are created and what slows down reactions.
This knowledge is necessary for the industrial production of various chemicals, such as:
fertilizersmedications, and home cleaners.Learn more about rate of reaction:
https://brainly.com/question/24795637
#SPJ1
Which statement best describes physical properties?
A. Physical properties behave identically for all matter under the same conditions.
B. Physical properties can be observed without changing the identity of a substance. C. Physical properties are observed by seeing how a substance reacts with other substances.
D. Physical properties cause atoms and molecules to change structure when substances are mixed.
Answers
Answer:B
Explanation:
Drew added yeast and sugar to lukewarm water and placed it in a flask. He
stretched a balloon over the opening of the flask. After several minutes, he noticed
small bubbles rising along the side of the flask as the balloon slowly began to inflate.
Drew concluded that -
A)a physical change was occurring
B) the bubbles indicated a chemical change
C) the water surger and yeast were products of a chemical reaction
D) the strong smell was evidence of a physical changer
Answers
Drew added yeast and sugar to lukewarm water and placed it in a flask. Drew concluded that the bubbles indicated a chemical change. The correct option is B)
What are chemical changes?A chemical change is a change when the identity of a substance changes, and it converts into a new substance with new characteristics and a new identity.
When iron comes in contact with oxygen, it changes into rust, and it is a chemical change. A chemical change is a transformation in which the substance's chemical composition is altered through atom rearrangement. Atomic alterations cause changes in the molecular structure.
In the reaction, when yeast reacts with lukewarm water it forms bubbles, and in cold water, yeast does not perform well.
Therefore, the correct option is B) the bubbles indicated a chemical change.
To learn more about chemical changes, refer to the link:
https://brainly.com/question/29715141
#SPJ2
Question 14 PM2.5 is defined as ________
- the mass concentration of particles in the air less than or equal to 2.5 micrometers in diameter. - the mass concentration of particles in the air equal to 2.5 micrometers in diameter. - the mass concentration of particles in the air greater than or equal to 2.5 micrometers in diameter. Question 15 Carbon dioxide (CO2) is a criteria air pollutant. - True - False Question 16 Roughly percent of emissions of carbon monoxide in Santa Clara County come from mobile sources (select the choice closest to the correct answer). - 50 - 75 - 25 Question 17
The term "photochemical smog" is most synonymous with which of the following criteria air pollutants? - lead (Pb) - carbon monoxide (CO) - sulfur dioxide ( SO2) - ozone (O3) Question 18 "Attainment" of ambient air quality standards requires that measured concentrations at all monitoring stations within an air district are below ambient air standards. - True - False
Answers
: PM2.5 is defined as the mass concentration of particles in the air less than or equal to 2.5 micrometers in diameter.Question 15: False, carbon dioxide (CO2) is not considered a criteria air pollutant.
Question 16: The closest answer is 50%, but the exact percentage is not provided in the question.Question 17: The term "photochemical smog" is most synonymous with ozone (O3), which is a criteria air pollutant.Question 18: True, attainment of ambient air quality standards requires that measured concentrations at all monitoring stations within an air district are below ambient air standards.
Question 14 asks about the definition of PM2.5. PM2.5 refers to particulate matter with a diameter less than or equal to 2.5 micrometers. It represents the mass concentration of particles suspended in the air, which are small enough to be inhaled into the respiratory system and can have adverse health effects.
Question 15 states whether carbon dioxide (CO2) is a criteria air pollutant. Criteria air pollutants are a set of pollutants regulated by environmental agencies due to their detrimental impact on air quality and human health. However, carbon dioxide is not considered a criteria air pollutant because it does not directly cause harm to human health or the environment in the same way as pollutants like ozone or particulate matter.
Question 16 asks about the percentage of carbon monoxide (CO) emissions from mobile sources in Santa Clara County. While the exact percentage is not provided in the question, the closest answer option is 50%. However, it is important to note that the precise percentage may vary depending on specific local conditions and emissions sources.
Question 17 inquires about the criteria air pollutant most synonymous with the term "photochemical smog." Photochemical smog is primarily associated with high levels of ground-level ozone (O3). Ozone is formed when nitrogen oxides (NOx) and volatile organic compounds (VOCs) react in the presence of sunlight, creating a hazy and polluted atmospheric condition.
Question 18 addresses the concept of "attainment" of ambient air quality standards. To achieve attainment, measured concentrations of pollutants at all monitoring stations within an air district must be below the established ambient air quality standards. This ensures that the air quality in the given area meets the required standards for protecting human health and the environment.
Learn more about mass concentration here:- brainly.com/question/23437000
#SPJ11
what is the normal range of pH levels of blood and tissue fluids in the human bodywhat is the normal range of ph levels of blood and tissue fluids in the human body? what is the difference between a strong acid and a weak acid?
Answers
The normal range of pH levels in blood and tissue fluids in the human body is approximately 7.35 to 7.45. This range is slightly alkaline, indicating a slightly basic or basic condition.
A strong acid is a substance that completely dissociates in water, releasing a high concentration of hydrogen ions (H+). This results in a low pH value. Strong acids are highly reactive and can cause severe burns or damage. Examples include hydrochloric acid (HCl) and sulfuric acid (H2SO4).
A weak acid, on the other hand, only partially dissociates in water, releasing a lower concentration of hydrogen ions (H+). This results in a higher pH value compared to strong acids. Weak acids are less reactive and tend to be less harmful. Examples include acetic acid (CH3COOH) and carbonic acid (H2CO3).
The main difference between strong acids and weak acids lies in their degree of dissociation and the concentration of hydrogen ions they release when dissolved in water, which affects their acidity and pH value.
learn more about pH levels here:
https://brainly.com/question/2288405
#SPJ11
How much water is needed to dissolve 30g or Pb(NO3)2 at 40.0C answer the question? NO BOTS ALLOWED
Answers
Answer:
10 must be added
Explanation:
Write a balanced half-reaction for the OXIDATION of aqueous Hydrogen Peroxide (H2O2) to Gaseous Oxygen (O2) in basic aqueous solution. Be sure to ADD physical state symbols where appropriate.
Answers
The balanced half-reaction:
The reactant H₂O₂(aq) is oxidized to form O₂(g) and 2OH-(aq).
The hydroxide ions (OH-) act as the reducing agent, accepting the electrons lost by hydrogen peroxide.
The balanced half-reaction for the oxidation of aqueous hydrogen peroxide (H₂O₂) to gaseous oxygen (O₂) in a basic aqueous solution can be represented as follows:
H₂O₂(aq) -> O₂(g) + 2OH-(aq)
This equation represents the oxidation of hydrogen peroxide, where it loses electrons and forms oxygen gas. In the basic solution, hydroxide ions (OH-) are present to balance the charges in the reaction.
Therefore,
In the balanced half-reaction:
The reactant H₂O₂(aq) is oxidized to form O₂(g) and 2OH-(aq).
The hydroxide ions (OH-) act as the reducing agent, accepting the electrons lost by hydrogen peroxide.
Know more about half-reaction:
https://brainly.com/question/18403544
#SPJ12
Some of the brine is encapsulated within ice crystals, but most is trapped in the spaces between neighboring crystals. When air temperature falls below 0°C, the brine migrates downward, toward the higher water temperatures below. Eventually, the high-density brine drains into the water beneath the ice. In the process, the sea ice freshens while the salinity of the underlying water. and becomes
a increases less dense
b
increases
more dense
c. decreases less dense
d. decreases
more dense
Answers
The high-density brine drains into the water beneath the ice and in the process, the sea ice freshens while the salinity of the underlying water decreases, becoming less dense (Option C).
Sea ice is usually less salty than the ocean water it freezes from. During the process of ice formation, salt in the ocean water is expelled from the ice as it grows; most of the salt is ejected into the ocean but some remain trapped inside pockets of brine within the ice. When the air temperature falls below the freezing point of seawater (usually around -1.8 °C), water molecules start to form ice crystals, which grow and aggregate into a solid sheet of ice.
During this process, the salt rejected by the growing ice also accumulates, causing the salinity of the remaining brine to increase. Some of the brine is encapsulated within ice crystals, but most are trapped in the spaces between neighboring crystals.
Thus, the correct option is C.
Learn more about brine: https://brainly.com/question/14667236
#SPJ11
What is the major organic product obtained from the following reaction? 1. nano2 hcl 2. hbr cubr
Answers
The major organic product obtained from the given reaction sequence is 2-bromo-1-chlorobenzene.
In the first step of the reaction sequence, NaN02 (sodium nitrite) and HCl (hydrochloric acid) are used to convert an amine group (-NH2) to a diazonium salt (-N2+). This step is known as diazotization. The specific compound involved in the reaction is not mentioned in the question, so we'll assume it is an aromatic amine.
In the second step, HBr (hydrobromic acid) and CuBr (copper(I) bromide) are added. The diazonium salt reacts with HBr to form a bromoarene compound. The CuBr serves as a catalyst for the reaction.
The product obtained from the reaction sequence is 2-bromo-1-chlorobenzene. The amine group (-NH2) in the starting compound is replaced by a bromine atom (-Br) through the diazotization and bromination reactions.
It's important to note that without specific details about the starting compound, the exact product cannot be determined. However, based on the given reaction sequence, 2-bromo-1-chlorobenzene is the expected major organic product.
Learn more about 2-bromo-1-chlorobenzene from the given link https://brainly.com/question/14469260
#SPJ11.
which change is always larger upon adding a solute, the change in freezing temperature or the change in boiling temperature?
Answers
change in boiling point temperature is always larger upon adding a solute.
When the vapor pressure of a liquid reaches the pressure of the gas above it, the liquid boils. The temperature at which a liquid will boil decreases with decreasing gas pressure above the liquid.
Up until the critical pressure, the boiling point rises with an increase in pressure, at which time the characteristics of gas and liquid are similar. Beyond the critical point, the boiling point cannot rise. Similar to how the triple point is attained, the boiling point falls with falling pressure. It measures the point at which a chemical boils, to put it simply. A higher boiling point denotes more intermolecular forces and, thus, less vapor pressure, similar to a higher melting point.
to learn more about a boiling point here;
https://brainly.com/question/4463307
#SPJ4
Which term describes difference between the actual price of inputs and the standard price of inputs? Select one: A. Standard cost variance B. Price variance C. Inflation index adjustment D. Materials price index
Answers
So the correct option is B. Price variance. Price variance term describes difference between actual price of inputs and the standard price of inputs.
Price variance refers to the difference between the actual price paid for inputs and the standard price set for those inputs. It is a measure of the discrepancy between the expected or budgeted cost or actual cost of acquiring materials, goods, services. Price variance helps in evaluating the effectiveness of procurement activities and assessing the impact of price fluctuations on overall costs. A favorable price variance indicates that inputs were obtained at a lower cost than expected, while an unfavorable price variance suggests higher costs than anticipated.
Learn more about Price here;
https://brainly.com/question/24872937
#SPJ11
Select the correct answer.
An iron nail is made up of particles. What is true about the particles?
A.They move all over the place.
B.The force of attraction between them is small.
C.The spacing between them
is large.
D. They stay in place and vibrate.
Answers
What is true about the particles?
D. They stay in place and vibrate.
the heat energy absorbed or released during a chemical reaction is known as choose... , or δh. a reaction that is exothermic, or releasing energy, will have a δh value that is choose... . a reaction that is endothermic, or absorbing energy, will have a δh value that is choose... .
Answers
The heat energy absorbed or released during a chemical reaction is known as enthalpy change or ΔH.
A reaction that is exothermic, or releasing energy, will have a ΔH value that is negative.
A reaction that is endothermic, or absorbing energy, will have a ΔH value that is positive.
Enthalpy change is defined as the heat exchanged between the system and the surroundings at a constant pressure.
It is denoted by ΔH, which is equal to the change in heat (q) of the system at a constant pressure.
The value of enthalpy change (ΔH) determines whether a reaction is exothermic or endothermic.
When the value of ΔH is negative, the reaction is exothermic, releasing energy in the form of heat to the surroundings.
When the value of ΔH is positive, the reaction is endothermic, absorbing energy from the surroundings.
To know more about enthalpy change visit;
https://brainly.com/question/31663014
#SPJ11
Use the balanced equation to solve the problem.N2 + 3H22NH323.0g NH3 are made.How many liters of H₂ gas reacted at Stp? L
Answers
By using the ideal gas law to get volume we have"
\(V=\frac{nRT}{P}\)Where v is volume, T is temperatute, n is number of moles, R is the molar gas constant and P is pressure. At STP P= 101,325 Pa, T= 273.15 K and R= 8.314 J/mol K
\(\begin{gathered} \frac{RT}{P}=0.022414cm^3mol^{-1} \\ \\ V=0.0022414n \end{gathered}\)We must first convert mass to moles:
\(\begin{gathered} mole=\frac{mass}{molecular\text{ }mass} \\ mole=\frac{23.0g}{17.0g\text{ }mol^{-1}} \\ \\ mole=1.35 \end{gathered}\)\(To\text{ }determine\text{ }the\text{ }moles\text{ }of\text{ }H2\text{ }gas\text{ }reacted\text{ }we:\frac{2}{3}\times1.35=0.87\text{ }mol\)By substituting this value into the ideal gas law we have:
\(\begin{gathered} V=0.0022414cm^3mol^{-1}\times0.87mol \\ V=0.0019502cm^3 \\ \\ V=1.9502\times10^{-6}L \end{gathered}\)1.9502e-6L of H2 gas reacted at STP
What is a trade-off?
Don't mind the highlighted yellow circle

Answers
Answer:
a new scientific discovery that benefits the environment
which diagram represents an electrolytic solution
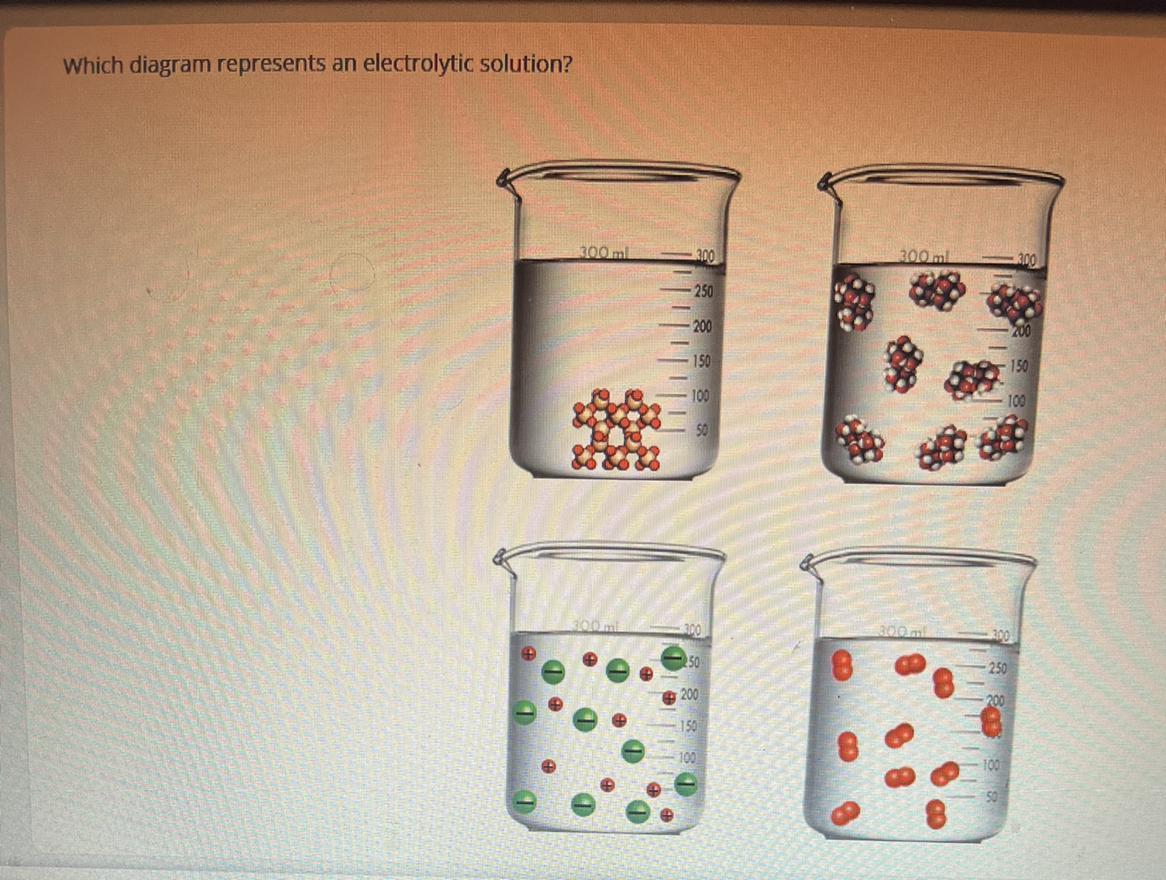
Answers
Answer:
Image
Explanation:
An electrolytic solution is a solution that has the ability of conducting electricity. Electrolytic solutions contain ions in which it conducts electricity from those ions. Ions are charged atoms. This solution (refer to image) has ions, as represented by the + and - symbols.

What is the standard potential, e∘celle∘cell, for this galvanic cell? use the given standard reduction potentials in your calculation as appropriate
Answers
0.56 V is the standard potential, e∘cell∘, for this galvanic cell.
The standard reduction potential can be calculated by subtracting the standard reduction potential for the reaction occurring at the cathode from the standard reduction potential for the reaction occurring at the anode. The minus sign is necessary because oxidation is the polar opposite of reduction. The total cell potential can be calculated using the formula E0cell=E0red+E0oxid. Step two is to find a solution. Before the two reactions may be integrated, the number of electrons gained in the reduction must match the number of electrons lost in the oxidation
Learn more about the standard potential here brainly.com/question/19036092
#4204.
The standard potential for the given galvanic cell is 0.477 V
What is electrode potential?The electrode potential is the electromotive force of a galvanic cell built using a standard reference electrode and another electrode whose potential is to be found.
There are two types of electrode potential
Oxidation potential - The potential associated with oxidation reaction is known as oxidation potential
Reduction potential - The potential associated with reduction reaction is known as reduction potential
At the anode, oxidation occurs
\(Sn(s)\rightarrow Sn^{2+}(aq)+2e^-\)
At the cathode, reduction occurs
\(Cu^{2+}(aq)+2e^-\rightarrow Cu(s)\)
\(E^o_{cell} =E^o_{cathode} -E^o_{anode}\)
= 0.337 - (-0.140)
= 0.477 V
Thus, The standard potential for the given galvanic cell is 0.477 V
Learn more about electrode potential:
https://brainly.com/question/17362810
#SPJ4
Disclaimer: The question was given incomplete on the portal. Here is the complete question
Question: What is the standard potential, E∘cell, for this galvanic cell? Use the given standard reduction potentials in your calculation as appropriate.
\(Sn^{2+}(aq)+2e^-\rightarrow Sn(s)\), E°red=−0.140 V
\(Cu^{2+}(aq)+2e^-\rightarrow Cu(s)\), E°red=+0.337 V
a solution contains 0.0420 m ca2 and 0.0980 m ag . if solid na3po4 is added to this mixture, which of the phosphate species would precipitate out of solution first?
Answers
A solution contains 0.0420 M Ca²⁺ and 0.0980 M Ag⁺ . If solid na3po4 is added to this mixture, the phosphate species that precipitate out of solution first is Ca₃(PO₄)₂.
The molarity of [Ca²⁺] = 0.0420 M
The molarity of [Ag⁺] = 0.0980 M
The dissociation is as :
Ag₃PO₄ ---> 3Ag⁺ + PO³⁻₄
Solubility product constant Ksp of Ag₃PO₄ = 8.89 × 10⁻¹⁷
Ksp = [ Ag³⁺] [ PO³⁻₄]
[ PO³⁻₄] = 1.0 × 10⁻¹³
The solubility product constant of Ca₃(PO₄)₂ = 2 × 10⁻³²
Ca₃(PO₄)₂ ---> 3Ca²⁺ + 2PO₄³⁻
[PO₄³⁻] = 4.9 × 10⁻¹⁵
Thus, Ca₃(PO₄)₂ will precipitate the first.
To learn more about solubility product here
https://brainly.com/question/1419865
#SPJ4
What volume of water is used to prepare a 3.5 M solution if 45 grams of Na2CO3 is used?
Answers
Answer:
121.3 ml
Explanation:
Mole wts
Na 22.989 x 2
C 12.011
O 15.999 x 3 total = 105.986
45 grams is 45/105.986 of a mole
45/105.986 in one liter would be .424584 M
.424585 mole / x liter = 3.5
x = .1213 liter = 121.3 ml
Engineering Question 15 of 30 Which of the following devices is used for atomizing and vaporizing the fuel before mixing it with air in varying proportions? O Spark plug O Carburetor O Flywheel Govern
Answers
The primary goal of the carburetor is to atomize and vaporize the fuel before mixing it with air in varying proportions, making it the device that is used for atomizing and vaporizing the fuel before mixing it with air in varying proportions.
The device that is used for atomizing and vaporizing the fuel before mixing it with air in varying proportions is Carburetor. A carburetor is a device that blends air and fuel for an internal combustion engine. It is often located on the top of an engine on a direct engine-to-carburetor link, and it controls how much air and fuel are mixed.The carburetor must also supply the engine with a spark plug to ignite the fuel/air mixture in each cylinder.
The carburetor must provide a fuel/air mixture that is consistent with the engine's changing demands, which vary with engine speed, load, and temperature. A carburetor is responsible for enriching the fuel/air mixture when the engine is cold and for leaning the mixture as the engine warms up. As well, it is also responsible for regulating the fuel/air mixture at part-throttle levels, where the engine spends most of its time when driving.
When an engine is running at full throttle, it is operating at wide-open throttle (WOT), and the carburetor provides the richest fuel/air mixture possible.The carburetor, like most engine systems, is a complex and sensitive device that must be correctly tuned to perform properly. The primary goal of the carburetor is to atomize and vaporize the fuel before mixing it with air in varying proportions, making it the device that is used for atomizing and vaporizing the fuel before mixing it with air in varying proportions.
To know more about Carburetor visit :
https://brainly.com/question/32239564
#SPJ11
What type of bond forms when the sulfur atoms in two adjacent protein chains are joined together?what type of bond forms when the sulfur atoms in two adjacent protein chains are joined together?
Answers
Disulfide bond forms when the sulfur atoms in two adjacent protein chains are joined together.
What kinds of side bonds are there in proteins?When the sulfur atoms in two adjacent protein chains come together, strong chemical side bonds form. Weak physical side bonds are caused by the attraction of opposite electrical charges. Polypeptide chains that are coiled. Chemical bonds that connect amino acids end to end in long chains.
How do peptides join together to form proteins?These peptide subunits can form complex structures by bonding with other peptides. Chemical bonds of various types hold proteins together and bind them to other molecules. Examine the chemical bonds that are responsible for protein structure. A protein's primary structure is made up of amino acids linked together in a chain.
Learn more about amino acids here:-
https://brainly.com/question/25188678
#SPJ4
34.8 g of Na₂O are used to form a solution with a volume of 0.50 L. What is the molarity?
Answers
Answer:
Molarity = 1.12 mol/L
Explanation:
To make an aqueous solution of Na₂O, the concentration will be calculated by: concentration (c) (or molarity) = number of moles present (n) ÷ volume needed (V) (in litres)
since we don't have moles, we can calculate moles by:
number of moles (n) = mass present (m) (in grams) ÷ molar mass (M) (in grams per mole), which we can find using a standard IUPAC Periodic Table
∴ n(Na₂O) = m/M = 34.8/(22.99×2+16.00) = 0.56147 mol
Now we have the number of moles present, we can calculate concentration:
∴ c(Na₂O) = n/V = 0.56147/0.50L = 1.12 mol/L
TASK 1 Metals A, B and B are given. Metal A is a stronger reducing agent than B. Metal B can displace metal 5 from solutions of its salts, but does not react with solutions of salts of A. Arrange metals A, B and In reducing their reduction capacity. We select om POAM two triplets meta- Au, koumo meet these conditions.
Answers
Here, the metal A displaces metal B from its solution. Hence, the metal A has lower positive reduction than B. Hence, the reduction potential of B is higher and it is less strong reducing agent.
What is reduction potential ?The reduction potential of a metal electrode is the measure of the tendency of it to lose or gain electrons and is the equilibrium potential difference developed due to separation of charges at the metal - solution interface when a metal is kept in contact with solution of its own.
The higher the negative reduction potential, greater the reducing capacity of the metal. Hence, metals with higher positive potential are easily reducing or they are strong oxidizing agents.
The metals with strong reducing power displaces other metals with lower reducing power from their solution. Here, A is strong reducing agent. Hence, it can displace B from its salt solution and B reduces to its metallic form.
Therefore, the order of A and B in reducing their reduction capacity is A>B.
Find more on reduction potential:
https://brainly.com/question/23881200
#SPJ1
Semiconductors are more effective than metals as converters of solar radiation into electricity because?
Answers
Semiconductors show the property of both the conductor and insulator. They are more effective than metals as their valence electron can free flow by the sun's energy. Thus, option B is correct.
What are semiconductors?Semiconductors are substances and materials that exhibit intermediate properties of conductors and insulators. They are solid materials that can conduct electrical charges due to the added impurity or change in temperature.
They have a valence electron in their outer shell that can absorb the energy from the sun to get excited. This leads to the flow of the charge in the semiconducting substance more easily.
Therefore, option B. the semiconductors can absorb the extra energy is correct.
Learn more about semiconductors here:
https://brainly.com/question/18132856
#SPJ4
Your question is incomplete, but most probably your full question was, Semiconductors are more effective than metals as converters of solar radiation into electricity because
A. it is easier for the atoms of a semiconductor to absorb solar energy and to move from place to place within the solid, thereby conducting electricity.
B. the energy contained in sunlight gives the valence electrons in the semiconductor atoms the extra energy they need to "flow" throughout the solid.
C. metals melt much too easily under direct sunlight and, as liquids, lose their ability to conduct electricity.
D. solar radiation has sufficient energy to knock electrons completely off the metal surface, making it impossible for the electrons to "flow" from atom to atom and conduct electricity.
solid added to liquid and the solid floats down to bottom of container physical or chemical change?
Answers
Answer:
Physical change
Explanation:
Which is the correctly balanced chemical equation for the reaction of KOH and H2SO4? Which is the net ionic equation for the reaction?
Answers
Answer:
See explanation
Explanation:
A balanced chemical reaction equation has the same number of atoms of each element on both sides of the reaction equation.
Hence, for the reaction between KOH and H2SO4, the balanced chemical reaction equation is;
H2SO4(aq) + 2KOH(aq) ---------> K2SO4(aq) + 2H2O(l)
Complete ionic equation;
2H^+(aq) + SO4^2-(aq) + 2K^+(aq) +2OH^-(aq) -------> SO4^2-(aq) + 2K^+(aq) + 2H2O(l)
Net ionic equation;
2H^+(aq) + 2OH^-(aq) -------> 2H2O(l)
o-linked oligosaccharides are commonly attached to the —oh group of
Answers
O-linked oligosaccharides are commonly attached to the —oh group of amino acids.
Glycosylation is a post-translational modification of proteins in which glyco-linked oligosaccharides are added to proteins. This process is found in eukaryotes, primarily in the endoplasmic reticulum and Golgi apparatus, and involves the enzymatic attachment of sugar units to specific sites on a protein molecule.
These short chains of saccharide sugar residues, called glycosyl sidechains, are examples of glycoconjugates which are covalently attached to hydroxyl groups on the protein.
The most common types of glycosylation sites are the N-linked glycosylation sites, which have an attached sugar group, often an N-acetylglucosamine or N-acetylmannosamine connected to an asparagine sidechain, and the O-linked glycosylation sites, which have a sugar group, usually a galactose or mannose sugar, attached to a hydroxyl group on serine, threonine or hydroxylysine residues.
know more about oligosaccharides here
https://brainly.com/question/33448934#
#SPJ11
what will happen when the earth stop rotating
Answers
Answer:
The momentum would send things flying eastward
Explanation:
Since earth is rotating at around a speed of thousand miles per hour you could expect things to fly eastward.