Vapor pressure of any liquid _______. A. increases with increases in temperature B. increases with decreases in temperature C. is not affected by changes in temperature D. increases with increases in volume of the liquid E. None of the Above
Answers
Answer:
increase with increases in temperature
Explanation:
please mark me brainleast
Related Questions
how can we separate the sugar from sugar solution
Answers
Why would it be unreasonable for an amendment to the clean air act to call for 0%
pollution emissions from cars with combustion engines?
Answers
It would be unreasonable for an amendment to the clean air act to call for 0% pollution emissions from cars with combustion engines because practically it is not possible to have 0% pollution emission.
The CAA was amended in 1965 with the Engine Vehicle Air Contamination Control Act (MVAPCA) which gave the Slash Secretary power to set government guidelines for vehicle emanations as soon as 1967.
In 1963, The Clean Air Act (CAA) was passed. It was an augmentation of Air Pollution Control Act, 1955, . The main idea behind this act was to empower the national government through US General administration under the division of Wellbeing, Government assistance and schooling, and to extend support towards innovative work and minimizing pollution.
To know more about clean air act here
https://brainly.com/question/30057213
#SPJ4
If salinity does not change, how does seawater density change as temperature changes?
Answers
The density of water decreases as it becomes warmer, more space. When comparing two samples same salinity of water, sample with the higher temperature will have more volume and hence be less dense.
What causes the salinity?The accumulation of salt from rainfall over a long period of time or the weathering of rocks are two examples of natural processes that contribute to primary salinity.
How salinity is measured?By running an electric current between a salinity meter's two electrodes in a sample of soil or water, it is possible to determine the salinity of both water and soil. The quantity and make-up of dissolved salts affect the electrical conductivity, or EC, of a soil or water sample.
To know more about Salinity visit:
https://brainly.com/question/14575146
#SPJ13
calculate the pressure inside a tube given that the tubes colume is 5.0l its tempreature is 23 c and it contains 0.010 of hydrogen gas
Answers
Hence, the pressure inside the tube is 2.4x10-5 atm
What is Pressure?
A key idea in physics is pressure, which is measured as the amount of force per unit area acting perpendicular to a surface. The pascal (Pa), or one newton per square metre, is the SI unit for pressure. [1]
P=FA, with
Pressure is P.
A is the area of the applied force, and F is the force that is applied normal (perpendicular) to the surface.
Volume of the tube (V) = 5.0 L
Temperature (T) = 23 °C = (23 + 273.15) K = 296.15 K
Mass of hydrogen gas (m) = 0.010 mg = 0.010 x 10-3 g
Molar mass of H2 (M) = 2x1.00784 = 2.01568 g/mol
so, mole of H
2
(n) = 5.0x10
-5
mol
R = Gas constant = 0.082057 L-atm/mol-K
Now, using the ideal gas equation, we can find the pressure (P) inside the tube.
Ideal Gas Equation: PV = nRT
Hence, the pressure inside the tube is 2.4x10-5 atm
Learn more about Force from given link
https://brainly.com/question/28012687
#SPJ4
Answer:A key idea in physics is pressure, which is measured as the amount of force per unit area acting perpendicular to a surface. The pascal (Pa), or one newton per square metre, is the SI unit for pressure. [1]
P=FA, with
Pressure is P.
A is the area of the applied force, and F is the force that is applied normal (perpendicular) to the surface.
Volume of the tube (V) = 5.0 L
Temperature (T) = 23 °C = (23 + 273.15) K = 296.15 K
Mass of hydrogen gas (m) = 0.010 mg = 0.010 x 10-3 g
Molar mass of H2 (M) = 2x1.00784 = 2.01568 g/mol
so, mole of H
2
(n) = 5.0x10
-5
mol
R = Gas constant = 0.082057 L-atm/mol-K
Now, using the ideal gas equation, we can find the pressure (P) inside the tube.
Ideal Gas Equation: PV = nRT
Hence, the pressure inside the tube is 2.4x10-5 atm
Explanation:
Which of these is an extensive property of a substance?
O color
O hardness
O malleability
O volume
Answers
Answer:
volumeMassExplanation:
Extensive properties are ;
mass volume,Intensive properties are ;
density colour,Volume is an extensive property of a substance.
Which is an extensive property of a substance?An extensive property is a property that depends on the amount of matter in a sample. Mass and volume are examples of extensive properties. An intensive property is a property of matter that depends only on the type of matter in a sample and not on the amount.
Is density an extensive property?Density is an intensive property because there is a narrow range of densities across the samples. No matter what the initial mass was, densities were essentially the same. Since intensive properties do not depend on the amount of material, the data indicate that density is an intensive property of matter.
Learn more about the extensive property here https://brainly.com/question/17337720
#SPJ2
is the measure of this flow? its for thermal energy transfer interactive
Answers
A chemist accidentally leaves an open beaker containing 300.0 mL of a 0.125 M NaCl(aq) solution on a lab bench. They return a few weeks later to find that the volume of the solution has decreased to 50.0 mL. What is the molarity of this partially evaporated solution, assuming the solute does not evaporate
Answers
The molarity of the partially evaporated solution is 0.75 M.
A chemist accidentally leaves an open beaker containing 300.0 mL of a 0.125 M NaCl(aq) solution on a lab bench. They return a few weeks later to find that the volume of the solution has decreased to 50.0 mL. What is the molarity of this partially evaporated solution, assuming the solute does not evaporate?
To solve this problem, we will follow these steps:
1. Calculate the moles of solute (NaCl) in the initial solution
2. Assume the moles of solute remain constant after evaporation
3. Calculate the molarity of the partially evaporated solution
Step 1: Calculate the moles of solute in the initial solution
moles of solute = Molarity × Volume
moles of NaCl = 0.125 M × 300.0 mL = 0.125 M × (300.0 mL ÷ 1000 mL/L) = 0.0375 moles
Step 2: Assume the moles of solute remain constant after evaporation
As stated in the question, the solute (NaCl) does not evaporate. Therefore, the moles of NaCl remain the same at 0.0375 moles.
Step 3: Calculate the molarity of the partially evaporated solution
Molarity = moles of solute ÷ Volume
Molarity of NaCl = 0.0375 moles ÷ (50.0 mL ÷ 1000 mL/L) = 0.0375 moles ÷ 0.050 L = 0.75 M
So, the molarity of the partially evaporated solution is 0.75 M.
To know more about chemicals visit :-
https://brainly.com/question/29886197
#SPJ11
In a 1.0×10^−6M solution of Ba(OH)2(aq) at 25 °C, arrange the species by their relative molar amounts in solution.
Greatest amount
least amount
Answer Bank: H2O, Ba(OH)2, OH^-, Ba^2+, H3O^+
Answers
Arranging the species by their relative molar amounts in a 1.0×10^−6M solution of Ba(OH)2(aq) at 25 °C:
Greatest amount: OH^-
Ba(OH)2
Ba^2+
H2O
H3O^-
In the given solution of Ba(OH)2(aq), the compound dissociates into its constituent ions, Ba^2+ and OH^-. The concentration of OH^- will be twice the concentration of Ba(OH)2 since each Ba(OH)2 molecule produces two OH^- ions. Therefore, OH^- will be present in the greatest amount.
Ba(OH)2 will be the next species in terms of molar amounts, followed by Ba^2+ since they are both present at half the concentration of OH^-. Water (H2O) does not participate in the chemical reaction and remains unchanged in terms of molar amounts. H3O^+ is not mentioned in the given compound Ba(OH)2 and is not present in this solution.
Therefore, based on the relative molar amounts, the arrangement of the species is as follows: OH^- (greatest amount), Ba(OH)2, Ba^2+, H2O, H3O^+ (least amount).
Learn more about molar: https://brainly.com/question/837939
#SPJ11
Does someone know the answers?

Answers
Answer:
b)sugar and water : Sedementation
d) salt and water : evaporation
How many Mn atoms are found in the following compound?
2MnO4

Answers
Answer:
2
Explanation:
Given compound:
2MnO₄
This means that the compound contains:
2 × 1 Mn = 2 Mn2 × 4 O = 8 O\(\rule[225]{225}{2}\)
Hope this helped!
~AH1807A sample has 0.2 moles of salt dissolved in 2 L of solution. What is the molarity
Don't put no random answer if you don't know it don't answer it please... TY :)
Answers
Molarity is the ratio of the moles and the volume of the solution in liters. The molarity of the 0.2 mole salt is 0.1 M.
What is molarity?The molarity of the solution is the molar concentration of the moles of the solute dissolved in a liter of solution. It is given as,
\(\rm Molarity (M) = \rm \dfrac{\text{moles of solute (n)}}{\text{volume (V) in liters}}\)
Given,
Moles (n) of the salt = 0.2 mol
The volume of the solution (V) = 2 L
Substituting values in the above equation:
\(\begin{aligned} \rm M &= \dfrac{0.2}{2}\\\\&= 0.1 \;\rm M\end{aligned}\)
Therefore, 0.1 M is the molarity of the sample.
Learn more about molarity here:
https://brainly.com/question/3947967
#SPJ1
explain how magnesium bromide would be formed from atoms from magnesium and bromide
Answers
Answer: the resulting compound to be neutral, two bromine anions must combine with one magnesium cation to form magnesium bromide (MgBr2).
Explanation: Because the reaction between the two is
Bromine reacts with magnesium to form magnesium brom and
how is magnesium bromide formed? Magnesium bromide can be synthesized by reacting hydrobromic acid with magnesium oxide and crystallizing the product. It can also be made by reacting magnesium carbonate and hydrobromic acids, and collecting the solid left after evaporation.
When bacteria reproduces asexually are the offspring uniform or diverse?
Answers
Bacteria reproduces asexually and produce genetically identical offspring. They produces a large number of offspring quickly and all will be uniform.
What is binary fission?Binary fission is the asexual method of reproduction in bacteria. Asexual reproduction creates clones of the original parent, which results in kids that are genetically identical to the parent.
Asexual reproduction allows for the rapid production of vast numbers of children by a single person. Asexual reproduction is a successful method of reproduction in a stable or predictable environment since all the children will be suited to that environment.
Asexually reproducing organisms may be at a disadvantage in an unstable or unpredictable environment since all of the progeny are genetically similar and might not have the genetic diversity to survive in new or changing settings.
Find more on binary fission:
https://brainly.com/question/7639952
#SPJ2
2. What changes when an ion is made from an atom?
Answers
Answer:
When ions are made of a single atom, such as Li+1, they are called monatomic ions
Explanation:
Any atom or molecule with a net charge, either positive or negative, is known as an ion. An ion consisting of a single atom is a monoatomic ion; an ion consisting of two or more atoms is referred to as a polyatomic ion.
A slightly edited Exercise 6 of Chapter 4 (Page 90) states:
(a) Calculate the energy needed to bring a cup of water (about 250 g) from 10°C to the boiling point (100°C for water). Then, find the time it takes to heat this water (c) in a 1-kg aluminum pan sitting on a 1,500-W electric stove burner that transfers 75% of its energy output to the water and the pan. Assume the pan, too, starts at 10°C and has to be heated to water’s boiling point.
Solution:
(a) To heat just the water requires energy Qw=mwcwΔT (Equation 4.3), where ΔT=100∘C−10∘C=90∘C:
Qw=0.25kg(4184Jkg∘C)90∘C=94,140J
(c) On the stove, we also have to heat the pan. Aluminum’s specific heat is ca=900Jkg∘C , from table 4.3, (because this is lower than cw, it is easier to heat aluminum than water).
To heat just the aluminum pan requires energy, Qa=macaΔT=1kg(900Jkg∘C)90∘C.
The total energy to heat the pan of water on the stove is increased because of the finite efficiency:
Qtotal=Qw+Qaes=94,140J+81,000J0.75=233,520J
The time it takes to heat the water depends on the stove’s power: power = energy per time, so
t=energypower=QtotalPs=233,520J1,500Js=155.68or156sonthestove
Question:
Find the time, in seconds, it takes to heat this water in a 1-kg steel pan sitting on a 1,500-W electric stove burner that transfers 75% of its energy output to the water and the pan. Assume the pan, too, starts at 10°C and has to be heated to water’s boiling point. Round your answer to the nearest whole second.
Answers
The time it takes to heat this water in a 1-kg steel pan sitting on a 1,500-W electric stove burner that transfers 75% of its energy output to the water and the pan is 90 seconds (rounded to the nearest whole second).
We need to calculate the time taken to heat the water in a 1-kg steel pan sitting on a 1,500-W electric stove burner that transfers 75% of its energy output to the water and the pan. The given information are as follows:
Specific heat of water, cw = 4184 J/kg °C
Specific heat of steel, cs = 450 J/kg °C
Energy supplied by the electric stove burner, P = 1,500 W (75% of which is transferred to the water and the pan)
Mass of water, mw = 250 g = 0.25 kg
Mass of steel pan, ms = 1 kg
Initial temperature of water and steel pan, T1 = 10 °C
Final temperature of water and steel pan (boiling point of water), T2 = 100 °C
Heat absorbed by the steel pan = Qs = ms × cs × (T2 - T1)Heat absorbed by the water = Qw = mw × cw × (T2 - T1)
Total heat absorbed by the water and the pan = Q = Qw + Qs = (0.25 × 4184 × 90) + (1 × 450 × 90) J= 94,140 + 40,500 J= 1,34,640 J
Time taken to heat the water and the pan = t = Q/P= 1,34,640 / 1,500 s= 89.76 or 90 s
Therefore, the time it takes to heat this water in a 1-kg steel pan sitting on a 1,500-W electric stove burner that transfers 75% of its energy output to the water and the pan is 90 seconds (rounded to the nearest whole second).
To know more about Mass, visit:
https://brainly.com/question/11954533
#SPJ11
which of the following should be classified as a mixture?
Helium
Water
Iron
Air
Answers
Answer:
I'm pretty sure it's A)Helium
Explanation:
Answer:
AIR
Explanation:
Need help!!! Also don't joke around please

Answers
urea, (nh2)2co, is used in plastics and fertilizers. it is also the primary nitrogen-containing substance excreted by humans. (a) which bonds in the molecule are polar and which are nonpolar? (b) which is the most polar bond in the molecule? which atom is the negative end of the bond dipole?
Answers
(a) All the bonds present in urea are polar.
(b) The oxygen atom will carry the partial negative charge at the end of the bond dipole, thus C=O is the most polar bond.
What is polar and non-polar bonds?When atoms share their electrons inequitably, polar covalent bonds are formed, whereas non-polar covalent connections are formed when atoms divide their electrons more evenly.The uneven electron sharing is caused by discrepancies in the electronegativities of the two atoms sharing the electrons
(a) All of the bonds in a urea molecule are polar. The compound's formula is [(NH₂)₂CO]. The bonds (C=O, N-H, and C-N) are all polar because to large variances in electronegativities. O has a higher electronegative potential than C, and N has a higher electronegative potential than C and H.
(b) All the bonds present in urea are polar, however, the most polar bond is the connection between carbon and oxygen due to its greater electronegativity than a nitrogen atom (N), thus C=O is the most polar bond.
As the atom that will attracts the electrons due to its electronegative nature, the oxygen atom will carry the partial negative charge at the end of the bond dipole.
To know more about polar bonds refer to:
https://brainly.com/question/545359
#SPJ1
If you put 100g of NaOH in cube form and 200g of NaOH in powered form which will react with HCl at a faster rate? Explain why
Answers
Answer:
200g of NaOH in powered form will react with HCl at a faster rate
Explanation:
200g of NaOH in powered form will react with HCl at a faster rate because it is in powdered form and it will have high surface area to volume ration and hence high reactivity and rate of reaction as compared to the cube NaOH.
Which property do transition metals have in common?
Answers
Explanation: google (:
transition metals are good conductors of heat and electricity and it can hammered into shape easily and have high melting points.
what is transition metal?Transition metal are the chemical elements which have valence electron and it can participate in chemical bonds formation process in two shells.
The position of the transition metals in periodic table is the middle portions of the long periods between the groups on the left-hand side and the groups on the right.
Most of the transition metals are hard, strong, and lustrous and it has high melting and boiling points, good conductors of heat and electricity.
Some of the elements are important technologically are titanium, iron, nickel, and copper as they are used in electrical technology.
Other transition metals are used in useful alloys, with one another and with other metallic elements, dissolve in mineral acids, platinum, silver, and gold are some Nobel transition metal.
Learn more about transition metals, here:
https://brainly.com/question/12843347
#SPJ2
Which type of scientist
studies everything
from the tiniest
organisms to human
beings?
Answers
Answer:
Biology
Explanation:
Biology is the study of all living beings. From the smallest algae or micro organism to the biggest living being is all covered under biology. How micro organisms live, how do they function and how they affect other living beings is studied in biology.
Answer:
Micro bioligist
Explanation: hope this helps
what is usually needed for a decomposition reaction to take place?
Answers
Energy input in the form of heat, light, or electricity is necessary for the majority of decomposition reactions.
Do elements always result from a reaction of decomposition?
Decomposition reactions usually produce discrete elements as their byproducts. An addition reaction occurs when a reactant splits into two or more products. This breakdown reaction is 2AI2O3 4AI + 3O2.
What is frequently required for a decomposition process to take place?
Energy input in the form of heat, light, or electricity is necessary for the majority of decomposition reactions. Only two elements can be found in binary compounds. When a binary chemical breaks down into its constituent parts, it undergoes the simplest type of breakdown reaction.
Learn more about binary compounds:
brainly.com/question/2951729
#SPJ4
As water starts to freeze, the molecules of water
A. decrease speed
B. move more freely
C. gain thermal energy
D. increase in size
Answers
Kinetic energy is an energy present in the object with motion. When the water freezes then the speed of the molecules decreases. Thus, option A is accurate.
What is the relation between kinetic energy and temperature?Kinetic energy is possessed by an object when it is in moving motion and because of the molecules. When the temperature decreases the movement of the molecule decreases.
The decrease in the kinetic energy results in a gain of potential energy and release of heat in the surrounding. The temperature and the kinetic energy are directly proportional and affect the speed of the molecules.
Therefore, the freezing of water results in decreased speed of the water molecule.
Learn more about kinetic energy here:
https://brainly.com/question/6068541
#SPJ1
Zinc reacts with Copper (II) chloride to produce Copper and Zinc chloride. -write chemical formula
Answers
Answer:
here's the answer hope it helps

Which of the following is a rechargable battery? Select the correct answer below: a. dry cell b. alkaline battery c. lithium ion battery d. These are all rechargable batteries.
Answers
The correct answer to your question is: c. lithium-ion battery. Lithium-ion batteries are rechargeable, making them suitable for various applications like electronics and electric vehicles. In contrast, dry cell and alkaline batteries are typically single-use and not rechargeable.
The correct answer to your question is option c. Lithium ion battery is a rechargeable battery that is commonly used in electronic devices. It is known for its high energy density, which means it can store more energy in a smaller size compared to other types of batteries. In contrast, dry cell and alkaline batteries are typically single-use and not rechargeable. This makes it popular in portable devices such as smartphones, laptops, and tablets. Lithium ion batteries typically last longer than other rechargeable batteries, making them a popular choice for consumers.
To know more about Lithium-ion batteries visit:
https://brainly.com/question/31115504
#SPJ11
If 85.0 grams of ethanol reacts, how many grams of carbon dioxide are produced?
Answers
Answer:
162.4g of CO2 are produced
Explanation:
The combustion of ethanol, C2H5OH, occurs as follows:
2C2H5OH + 7O2 → 4CO2 + 6H2O
Where 2 moles of C2H5OH produce 4 moles of CO2.
To solve this question we need to convert the mass of ethanol to moles. Then, the moles of ethanol to moles of CO2 using the balanced reaction and to mass:
Moles C2H5OH -Molar mass: 46.07g/mol-
85.0g * (1mol / 46.07g) = 1.845 moles C2H5OH
Moles CO2:
1.845 moles C2H5OH * (4mol CO2 / 2mol C2H5OH) = 3.69 moles CO2
Mass CO2-Molar mass: 44.01g/mol-:
3.69 moles CO2 * (44.01g/mol) =
162.4g of CO2 are producedWhich of the following is a physical property of matter
Answers
Answer: Physical properties of matter include color, hardness, malleability, solubility, electrical conductivity, density, melting point, and boiling point. For the elements, color does not vary much from one element to the next
Answer:
Physical properties of matter include;
evaporationcondensationsublimationfreezingmelting…and so on\(.\)
by titration, it is found that 24.7 ml of 0.179 m naoh(aq) is needed to neutralize 25.0 ml of hcl(aq). calculate the concentration of the hcl solution.
Answers
By titration, it is found that the concentration of the HCl solution is 0.175 M.
To solve this problem using titration, we need to use the balanced chemical equation for the neutralization reaction between NaOH and HCl:
NaOH + HCl → NaCl + H2O
From the equation, we know that 1 mole of NaOH reacts with 1 mole of HCl to produce 1 mole of NaCl and 1 mole of H2O. Therefore, we can use the following formula to calculate the concentration of the HCl solution:
M(HCl) = (M(NaOH) x V(NaOH)) / V(HCl)
Where:
M(HCl) = concentration of HCl solution
M(NaOH) = concentration of NaOH solution (given as 0.179 M)
V(NaOH) = volume of NaOH solution used (given as 24.7 mL or 0.0247 L)
V(HCl) = volume of HCl solution used (given as 25.0 mL or 0.0250 L)
Plugging in the values, we get:
M(HCl) = (0.179 M x 0.0247 L) / 0.0250 L
M(HCl) = 0.175 M
Therefore, the concentration of the HCl solution is 0.175 M.
Learn more about titration at https://brainly.com/question/27107265
#SPJ11
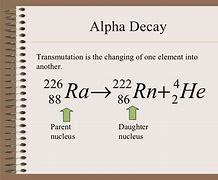
226 • Ra 4/2 He + 88 = ?
Answers
Answer: the answer for this question is in the pic
Explanation:
What are the solutions to problems caused by nitrogen gas in solution at certain depths?
Please Help!!!! 50 points
Answers
The solutions to problems caused by nitrogen gas in solution at certain depths is by discouraging deep diving.
What is Diving?
This involves moving around under water with the aid of special breathing equipment.
Limiting the depth of a dive should he encouraged as the maximum depth limit for a diver to use compressed air is 30 to 50 meters.
Read more about Diving here https://brainly.com/question/21503692