Answers
Theoretically, 14.29% of NaHCO3's carbon content is made up of carbon.
How many moles of NaCl are there to NaHCO3 in theory?The balancing coefficients for reaction 7.3 show that the mole ratio of reactant NaHCO3 to product NaCl is 1:1. This implies that 1 mole of sodium chloride should be created for every 1 mole of sodium bicarbonate that interacts.
Molar mass of NaHCO3 = (1 x molar mass of Na) + (1 x molar mass of H) + (1 x molar mass of C) + (3 x molar mass of O)
= (1 x 22.99 g/mol) + (1 x 1.01 g/mol) + (1 x 12.01 g/mol) + (3 x 16.00 g/mol)
= 84.01 g/mol
Next, we need to find the mass of carbon in one mole of NaHCO3.
Mass of carbon in NaHCO3 = 1 x 12.01 g/mol
= 12.01 g/mol
Finally, we can calculate the theoretical percent composition of carbon in NaHCO3:
Theoretical percent composition of carbon = (mass of carbon in 1 mol NaHCO3 ÷ molar mass of NaHCO3) x 100%
84.01 g/mol 12.01 g/mol = 100%
= 14.29%
To know more about Theoretically visit:-
https://brainly.com/question/14966377
#SPJ9
Related Questions
Define density. Plz help me
Answers
convert 8.42x10^8 mol/(kg*m^2) to mol/(g*cm^2)
Answers
Answer:
gguhg
Explanation:
no te es caso drama me están muy una las y y que las te
1 kg = 1000 g
1 m = 100 cm
Using these equations, 8.42x10^8 mol/(kgm^2) can be converted to 8.42x10^11 mol/(gcm^2).
Trend of atomic number and atomic size of the elements when we move from left to right in different periods of periodic table
Answers
Answer:
The atomic size decreases with an increase in atomic number when we move from left to right.
Explanation: Hope it helps you:))))))
Have a great day.
Which one is more basic, Li2O or Na2O? why?
Answers
Answer:
Na2O is more basic. The reasoning for this is because the basis of periodicity going from bottom to lower basicity is increase.
pOH of the 0.001M NaOH solution is
Answers
The pOH of the 0.001 M NaOH solution is approximately 3.
To determine the pOH of a solution, we need to know the concentration of hydroxide ions (OH-) in the solution.
In the case of a 0.001 M NaOH solution, we can assume that all of the NaOH dissociates completely in water to form Na+ and OH- ions. Therefore, the concentration of hydroxide ions in the solution is also 0.001 M.
The pOH is calculated using the equation:
pOH = -log[OH-]
Substituting the concentration of hydroxide ions, we have:
pOH = -log(0.001)
Using a calculator, we can evaluate the logarithm:
pOH ≈ 3
Therefore, the pOH of the 0.001 M NaOH solution is approximately 3.
Know more about hydroxide ions here:
https://brainly.com/question/28464162
#SPJ8
1
What is the name of Ba3(PO4)2?
O a. Tribarium Diphosphate
tof
O b. Triboron Diphosphate
O c. Boron Phosphate
O d. Barium Phosphate
Answers
Answer:
The answer is barium phosphate
A metal carbonate, XCO3 of mass 2.012 g was heated resulting in the formation of XO, a metal oxide and carbon dioxide with a mass of 0.855 g according to the reaction shown below: XCO3 (s) → XO (s) + CO2 (g) (Atomic mass of O-15.999 g/mol; H-1.008 g/mol; C-12.011 g/mol).
Answers
The metal X has an approximate molar mass of 42.36 g/mol and the metal is most likely calcium.
What is the molar mass of XCO₃?The molar mass of the metal carbonate XCO₃ and identify the metal X, we need to calculate the number of moles of XCO₃ and CO₂ using the given masses and molar masses.
The molar mass of CO₂ (carbon dioxide) is 12.011 g/mol (for carbon) + 2 * 15.999 g/mol (for oxygen) = 44.01 g/mol.
The number of moles of CO₂ can be calculated using the formula:
moles of CO₂ = mass of CO₂ / molar mass of CO₂
moles of CO₂ = 0.855 g / 44.01 g/mol
moles of CO₂ ≈ 0.01944 mol
Since the reaction stoichiometry is 1:1 between XCO₃ and CO₂, the number of moles of XCO₃ is also approximately 0.01944 mol.
molar mass of XCO₃ = mass of XCO₃ / moles of XCO₃
molar mass of XCO₃ = 2.012 g / 0.01944 mol
molar mass of XCO₃ ≈ 103.38 g/mol
The molar mass of XCO₃ is approximately 103.38 g/mol.
To determine the metal X:
molar mass of X = molar mass of XCO3 - molar mass of CO3
molar mass of X = 103.38 g/mol - (12.011 g/mol + 3 * 15.999 g/mol)
molar mass of X ≈ 42.36 g/mol
Metal X is most likely Calcium that has a molar mass of 40 g/mol
Learn more about molar mass at: https://brainly.com/question/837939
#SPJ1
A fossil that looks like a spiral or a rams horns
Answers
Answer:
Explanation:
ammonite
Answer:
Ammonite
Explanation:
These are some awesome fossils
Plz mark B R A I N L I E S T
Matter is anything the has mass and occupies space. True or False
Answers
Answer:
It's True.
Explanation:
Matter is anything that has mass and takes up space. Mass gives an object the property of weight and inertia (resistance to change in the motion of an object). There are four states of matter, solid, liquid, gas, and plasma.
physical proceses? 2. (a). Calculate the maximum work done when the pressure of 10g of hydrogen is reduced from 20atm to 10atm at a constant temperature of 273°K. If the gas behaves ideally, will there be a change in internal energy? Hence determine the value of q in the process.
Answers
W = nRT ln(V2/V1)
where W is the work done, n is the number of moles of gas, R is the gas constant, T is the temperature in Kelvin, and V1 and V2 are the initial and final volumes of the gas.
First, we need to calculate the initial and final volumes of the gas using the ideal gas law:
PV = nRT
where P is the pressure, V is the volume, and n, R, and T are as defined above. Rearranging this equation to solve for V, we get:
V = (nRT)/P
Using this equation, we can calculate the initial volume of the gas:
V1 = (10 g H2 / 2.016 g/mol) * (0.0821 L·atm/mol·K) * (273 K) / (20 atm) = 11.9 L
And the final volume of the gas:
V2 = (10 g H2 / 2.016 g/mol) * (0.0821 L·atm/mol·K) * (273 K) / (10 atm) = 23.8 L
Now we can plug these values into the formula for work:
W = (10 g H2 / 2.016 g/mol) * (0.0821 L·atm/mol·K) * (273 K) * ln(23.8 L / 11.9 L) = 98.8 J
If the gas behaves ideally, there will be no change in internal energy because the temperature is constant (ΔU = 0). Therefore, the value of q in the process is equal to the negative of the work done:
q = -W = -98.8 J.
asap! What is the chemical equation for the production of cesium vapor and molten calcium chloride from molten cesium chloride and molten calcium metal?
Answers
The equation of the combination of the cesium chloride and the calcium is shown in the image attached.
What is the equation?
We know that when we write the reaction equation, we are trying to show on a piece paper what is going on in the reaction system. In this case, we are going to have that there is in the system, the combination of molten cesium chloride and molten calcium metal.
In a reaction, we would have to ensure that the reaction equation is balanced. The implication of this is that the number of the atoms of each of the elements on both sides of the reaction equation must be the same.
Learn more about reaction equation:https://brainly.com/question/3588292
#SPJ1

Pls answer this question if you know it

Answers
Answer:
both waves are traveling at the same speed. the energy in the waves spread out as they travel through air...
Explanation:
that's their nature
PLEASE HELP IMMEDIATELY I NEED THE ANSWER NOT A HINT THANK YOU
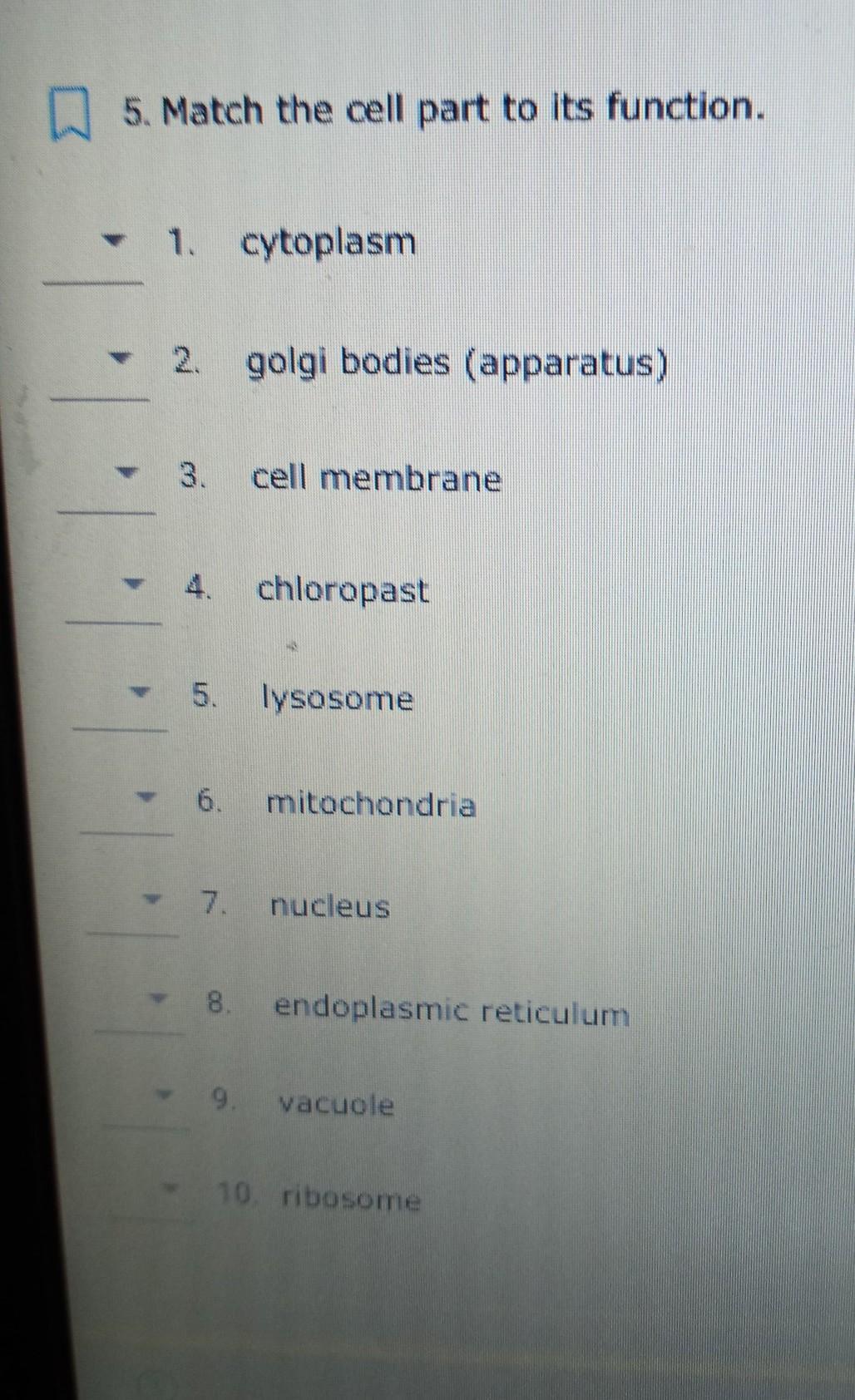
Answers
Cytoplasm: gel like environment which allows organelles to move about the cell
Golgi bodies: packages and ships materials out of the cell
Cell membrane: controls what goes in and out of the cell
Chloroplast: makes food for plant cells using sunlight
Lysosome: breaks down waste, food, and worn out cell parts
Mitochondria: breaks down food to release energy for the cell
Nucleus: contains the cell's DNA and is the control center of the cell
Endoplasmic reticulum: transports materials within cell; process lipids
Vacuole: stores water, waste and food
Ribosome: make proteins
Using this equation, m1v2=m2v2 , calculate the diluted molarity of 100 mL of a 0.5 M solution when 50 mL of
water has been added.
Answers
The molarity of the diluted solution is 0.33 M
From the question given above, the following data were obtained:
Molarity of stock solution (M₁) = 0. 5 M
Volume of stock solution (V₁) = 100 mL
Volume of diluted solution (V₂) = 100 + 50 = 150 mL
Molarity of diluted solution (M₂) =?The molarity of the diluted solution can be obtained by using the dilution formula as illustrated below:
M₁V₁ = M₂V₂0.5 × 100 = M₂ × 150
50 = M₂ × 150
Divide both side by 150
M₂ = 50 / 150
M₂ = 0.33 MTherefore, the molarity of the diluted solution is 0.33 M
Learn more: https://brainly.com/question/24625656
A titration required 42.00 mL of 0.150 M NaOH. How many moles of NaOH is this?
Answers
Answer:
0.006342moles
Explanation:
1000ml of NaOH contain 0.151moles
42ml of NaOH contain (42*0.151)/1000 moles
=0.006342moles
During which two processes does a substance absorb energy?
Answers
Answer:
melting and vaporization
Explanation:
what happens to energy when sally kicks a soccor ball
Answers
Answer:
it is turned into kinetic energy
Explanation:
Answer:
Kinetic energy is transferred from the leg to the soccer ball. or C
Explanation:
Hope this helps
1. why do the elements within a group of the periodic table have similar chemical properties?
Answers
Answer:
the elements in a group have similar electron configurations, an elements electron configuration determines its chemical properties, therefore members of a group in the periodic table have similar chemical properties. ... These metals have a single valence electron and are extremely reactive.
Explanation:
Answer:
Because they all have one or more atoms of the same type material
Explanation:
When zinc reacts with copper sulfate solution, zinc sulfate solution and copper are formed.(i) An experiment was carried out to measure the temperature change when zinc powder reactswith copper sulfate solution.initial temperature of copper sulfate solution = 20 °Cfinal temperature of mixture after the reaction = 46 °CExplain what the temperature readings show about the type of heat change that occurs duringthis reaction.
Answers
The temperature increase from 20 °C to 46 °C indicates that the reaction between zinc and copper sulfate solution is exothermic, with heat being released into the surroundings.
In the given reaction between zinc and copper sulfate solution, the temperature change can provide insights into the type of heat change occurring during the reaction. Based on the provided information, the initial temperature of the copper sulfate solution was 20 °C, and the final temperature of the mixture after the reaction was 46 °C.
The temperature increase observed in this reaction indicates an exothermic heat change. An exothermic reaction releases heat energy into the surroundings, resulting in a temperature rise. In this case, the reaction between zinc and copper sulfate solution is exothermic because the final temperature is higher than the initial temperature.
During the reaction, zinc displaces copper from copper sulfate to form zinc sulfate and copper metal. This displacement reaction is known as a single displacement or redox reaction. Zinc is more reactive than copper and therefore replaces copper in the compound.
The formation of new chemical bonds during the reaction releases energy in the form of heat. This energy is transferred to the surroundings, leading to an increase in temperature. The heat released is greater than the heat absorbed, resulting in a net increase in temperature.
The exothermic nature of this reaction can be explained by the difference in bond energies between the reactants and products. The breaking of bonds in the reactants requires energy input, while the formation of new bonds in the products releases energy.
In this case, the energy released during the formation of zinc sulfate and copper metal is greater than the energy required to break the bonds in copper sulfate and zinc.
For more such question on temperature visit:
https://brainly.com/question/4735135
#SPJ8
Describe four real life examples of the results of scientific investigation
Answers
The findings of scientific investigation can be seen in four real-world situations:
Pluto to not be a planet,compost,surgery,illness remediesExplain about the scientific investigation?Finding the answer to a topic through a variety of research techniques is the process of conducting a scientific investigation.
An investigation typically starts when a person analyzes their surroundings and poses questions they are unsure of the answers to. After that, they conduct additional observations or design an experiment to verify a theory. Observational research in science can involve, for instance, describing in-depth observations of a cell under a microscope. Some scientific studies are experimental; an illustration would be administering a chemical to a cell while observing changes in the conduct of the cell.Thus, the findings of scientific investigation can be seen in four real-world situations:
Pluto to not be a planet,compost,surgery,illness remediesTo know more about the scientific investigation, here
https://brainly.com/question/17571192
#SPJ1
full structural formula for CH3CH2CH(NH2)CH3
Answers
Answer: The structural formula can be represented as:
Explanation:
The question requires us to draw the structural formula for the compound CH3CH2CH(NH2)CH3.
The structural formula of a molecule gives us the location and type (simple, double or triple) of chemical bonds in the molecule.
To draw the structural formula of an organic compound, we must pay attention to the maximum number of bonds that carbon can make (4 bonds total) and place the atoms accordingly.
Considering the information above, the structural formula of the compound CH3CH2CH(NH2)CH3 can be represented as:
(In the image, the structural formula of the molecule is represented in black and below it, in blue, a similar representation to what was given by the question).
Note that all carbons represented are making a total of bonds and the heterogeneous group (NH2) is also bonded to a carbon in the main chain.


What is the formula for the polyatomic ion thiocyanate?
Answers
Answer:
Explanation:
Names - Formula
peroxide (O2 2−)
cyanide (CN−)
cyanate (OCN−)
thiocyanate (SCN−)
Convert 1,650,000 centimeters to kilometers.
Answers
Answer:
16.5
Explanation:
Answer:
is your answer is 16.5 km
How many kilojoules of heat are needed to raise the temperature of 10g of aluminum from 22 degrees C to 55 degrees C, if the specific heat of aluminum is .901 j/gc?
Answers
Answer:
name four agricultural inputs are subsidized by the government
0.297 kJ of heat is needed to raise the temperature of 10g of aluminum from 22 degrees Celsius to 55 degrees Celsius.
The specific heat is the amount of heat per unit mass required to raise the temperature by one degree Celsius.
It is a measure of how much energy it takes to raise the temperature of a substance. It is the amount of heat necessary to raise one mass unit of that substance by one temperature unit.
It is given by the formula -
Q = mcΔT
where, Q = amount of heat
m = mass
c = specific heat
ΔT = Change in temperature
Given,
mass = 10g
c = 0.901J/g⁰C
Initial temperature (T₁) = 22⁰C
Final Temperature (T₂) = 55⁰C
Q = mcΔT
= 10 × 0.901 × (55 -22)
= 297.33 J = 0.297 kJ
Learn more about Specific heat, here:
https://brainly.com/question/31608647
#SPJ1
A rigid 3.80 L sealed vessel contains 0.650 mol Ne, 0.321 mol Kr, and 0.190 mol Xe. Find the density of the mixture in g/L.
Answers
Answer:
17.09g/L
Explanation:
Density = total mass of elements/ volume
We need to find the mass of each mixture constituents using their molar mass:
mole = mass/molar mass
For Neon (Ne) which contains 0.650mol;
0.650 = mass/20.18
mass = 0.650 × 20.18
mass = 13.12g
For Krypton (Kr) which contains 0.321mol;
0.321 = mass/83.79
mass = 0.321 × 83.79
mass = 26.89g
For Xenon (Xe) which contains 0.190mol;
0.190 = mass/131.3
mass = 0.190 × 131.3
mass = 24.95g
Total mass = 13.12g + 26.89g + 24.95g = 64.96g
Density = total mass / volume
Density = 64.96g / 3.80L
Density of the mixture = 17.09g/L
Can metals take away electrons from non metals?
Answers
Answer:
Well no because if metals lose electrons, any non-metal sources/items gain electrons from the metal.
Answer:
Metals tend to lose electrons and non-metals tend to gain electrons, so in reactions involving these two groups, there is electron transfer from the metal to the non-metal
Explanation:
what mass of water is produced from 2.5g of glucose?
Answers
From the calculation, there are 1.5 g of water produced in the reaction.
What is an equation?An equation shows the chemical transformation that takes place in a reaction vessel. In this case, the equation is; C6H12O6 + 6 O2 --> 6 CO2 + 6 H2O.
Now;
Number of moles of glucose = 2.5g/180 g/mol = 0.014 moles
Since 1 mole of glucose produces 6 moles of water
0.014 moles of glucose produces 0.014 moles * 6 moles/ 1 mole
= 0.084 moles
Mass of water produced = 0.084 moles * 18 g/mol = 1.5 g of water
Learn more about glucose:https://brainly.com/question/2396657
#SPJ1
Read the descriptions below of two substances and an experiment on each. Decide whether the result of the experiment tells you the substance is a pure substance or a mixture, if you can.
Sample A is a solid yellow cube with a total mass of 50.0g. The cube is put into a beaker filled with 250.mL of water. The cube collapses into a small pile of orange powder at the bottom of the beaker. When this powder is filtered out, dried and weighed, it has a total mass of 29.9g If the experiment is repeated with 500.mL of water, the powder that's left over has a mass of 30.0g
Sample B is 100.mLof a clear liquid. The liquid is heated in a flask until it boils, which starts to happen at 66.2°C. As the liquid boils, the temperature continues to rise, until the last of the liquid boils away at 76.0°C..
Determine whether each is a pure substance, mixture, or can't decide.
Answers
Answer:
Sample A is a mixture
Sample B is a mixture
Explanation:
For sample A, we are told that the originally yellow solid was dissolved and we obtained an orange powder at the bottom of the beaker. Subsequently, only about 30.0 g of solid was recovered out of the 50.0g of solid dissolved. This implies that the solid is not pure and must be a mixture. The other components of the mixture must have remained in solution accounting for the loss in mass of solid obtained.
For sample B, we are told that boiling started at 66.2°C and continued until 76.0°C. The implication of this is that B must be a mixture since it boils over a range of temperatures. Pure substances have a sharp boiling point.
(01.01 LC)What is the body of scientific knowledge based on?
Guesses
Mysteries
Observations
Opinions
Answers
The body of scientific knowledge is based on different Observations (Option C).
What does observations mean in the scientific method?Observations in the scientific method are fundamental because it is the first step to raising scientific questions that may be explained through plausible hypotheses. Subsequently, hypotheses must be tested by experimental procedures.
In conclusion, the body of scientific knowledge is based on different Observations (Option C).
Learn more about observations in the scientific method here:
https://brainly.com/question/2505873
#SPJ1
A sample of metal has a mass of 24.64 g, and a volume of 5.91 mL. What is the density of this metal?
Answers
Answer:
\(\boxed {\boxed {\sf 4.17 \ g/mL}}\)
Explanation:
We are asked to find the density of a metal. Density is the mass per unit volume. It is calculated by dividing the mass by the volume.
\(\rho= \frac{m}{v}\)
The mass of the metal sample is 24.64 grams and the volume is 5.91 milliliters. We can substitute these values into the formula.
m= 24.64 g v= 5.91 mL\(\rho=\frac{24.64 \ g }{5.81 \ mL}\)
Divide.
\(\rho= 4.169204738 \ g/mL\)
The mass measurement has 4 significant figures the volume measurement has 3 significant figures. Our answer for density must match the least number of significant figures, which is 3.
For the number we calculated, that is the hundredth place. The 9 in the thousandth place tells us to round the 6 up to a 7.
\(\rho \approx 4.17 \ g/mL\)
The density of this metal is approximately 4.17 grams per milliliter.