Use the reaction and bond information to answer the question.
H2 + CO2 → CH2O2
Reactant bond energies: H–H is 432 kJ/mol, C=O is 799 kJ/mol
Product bond energies:
C–H is 413 kJ/mol, C=O is 745 kJ/mol
C–O is 358 kJ/mol, O–H is 467 kJ/mol
How much energy will be given off by the products?
1,570 kJ
1,983 kJ
1,238 kJ
1,516 kJ
Answers
\(\\ \bull\tt\longmapsto 413+745+358+467\)
\(\\ \bull\tt\longmapsto 1983KJ\)
Related Questions
The Ksp for LaF3 is 2 x 10^-19. What is the solubility of LaF3 in water in moles per liter?
Answers
The solubility of\(LaF_3\) in water is 3.04 x 10^-6 mol/L.
The solubility of \(LaF_3\) in water can be determined using the Ksp expression:
\(Ksp = [La^{3+}][F^-]^3\)
Where \([La^{3+}]\)and \([F^-]\) are the molar concentrations of the \(La^{3+}\) and \(F^-\) ions in the solution.
Since each \(LaF_3\) formula unit dissociates into one \(La^{3+}\) ion and three \(F^-\) ions, the molar solubility of \(LaF_3\) can be represented as x. Thus, the molar concentrations of \(La^{3+}\) and \(F^-\) ions in the solution can be written as x and 3x, respectively.
Substituting these values into the Ksp expression gives:
Ksp = x*(3x)^3 = 27x^4
Now, we can solve for x:
x = (Ksp/27)^(1/4)
= (2 x 10^-19 / 27)^(1/4)
= 3.04 x 10^-6 mol/L
For more question on solubility click on
https://brainly.com/question/24057916
#SPJ11
Determine the theoretical and percentage yield of the reaction below, if you started with 10 mL of the alcohol (C9H120; density = 0.900 g/ml.) and 10 ml of 12 Molar HCl to produce 5 mls of the product as a colorless liquid (C9H110; density - 0.800 g/mL)? Please show a step by step calculations for your answer.
Answers
The answer is 39.2%.
Solution:
\(Yield = \frac{Observed yield}{Theoritcal yield}*100\)
= \(\frac{4.0}{10.2} *100\)
= 39.2%.
Theoretical yields are calculated based on the stoichiometry of the chemical formula. Actual yield is determined experimentally. Percent yield is determined by calculating the ratio of actual yield to theoretical yield. how much usable product is obtained after processing; how much crude product is actually ordered.
However, in practice, the actual product yield is almost always lower than the theoretical yield. The actual yield is the amount of product actually obtained and the theoretical yield is the maximum possible yield. There are several reasons why percentage returns may not be 100%. This is because some other unexpected reaction occurred that did not produce the desired product.
Learn more about Theoretical yields here:- https://brainly.com/question/25996347
#SPJ4
determine the rate of the following reaction:2NO (g) + O2 (g) ---------> 2NO2 (g)The concentration of NO was 0.0500 M at t= 5.0 s and 0.0225 M at t= 650.0 s. What is the average rate of reaction during this time period?_________ M/s
Answers
We first verify that the reaction is balanced. We have 2 nitrogens and 4 oxygens from each of the reaction, so if it is balanced.
The average rate of the reaction (vm) will be:
\(v_m=-\frac{1}{StoichiometriCoefficient}\times\frac{\Delta\lbrack NO\rbrack}{\Delta t}\)Where,
Delta[NO] corresponds to the concentration difference
Delta[t] is the time difference
The Stoichiometric coefficient of NO is 2
We put the minus sign because it corresponds to a reactant.
We replace known data:
\(\begin{gathered} v_m=-\frac{1}{2}\times\frac{0.0225M-0.0500M}{650.0s-5.0s} \\ v_m=-\frac{1}{2}\times\frac{0.1750M}{645.0s}=-1.356\times10^{-4}\frac{M}{s} \\ \end{gathered}\)So, the average rate of reaction is -1.356x10^-4M/s
Question 1
This diagram shows Earth in four different positions during its yearly orbit around the sun. Which of the following accurately describes the position of the United States during the summer months?
Question 2
The diagram models 4 lunar phases. During which one is the tide the highest?
Question 3
An HR Diagram is shown below. A star that has a luminosity of 10^-2 is likely a…
Question 4
Earth's atmosphere blocks short wavelengths of the electromagnetic spectrum. Which telescopes DO NOT need to be placed in orbit around Earth to observe short-length radiation?
Question 5
A student models the relationship between the Earth and the Sun using string and a ball. Which of the following explains the relationship demonstrated?





Answers
Answer 1:
During the summer months in the northern hemisphere (where the United States is located), Earth is in position C, which is when the northern hemisphere is tilted towards the sun.
Answer 2:
The highest tide occurs during the full moon phase, which is represented by position C in the diagram.
Answer 3:
A star that has a luminosity of 10^-2 is likely a red dwarf.
Answer 4:
Telescopes that observe short-wavelength radiation, such as X-rays and gamma rays, do not need to be placed in orbit around Earth because these wavelengths are absorbed by the atmosphere. Therefore, telescopes that observe these wavelengths are typically placed in space, outside of Earth's atmosphere.
Answer 5:
The student is likely demonstrating the relationship between the Earth and the Sun's gravitational pull. The ball represents the Sun, and the string represents the gravitational force pulling the Earth towards the Sun. The demonstration shows how the Earth orbits the Sun due to this gravitational force.
What is gravitational force?Gravitational force is described as a force that exists between any two objects in the universe that have mass.
It is the force that causes objects with mass to be attracted to each other. The magnitude of the gravitational force between two objects depends on their masses and the distance between them.
Along with the electromagnetic force, the strong nuclear force, and the weak nuclear force, gravity is one of the four fundamental forces of the universe.
Sir Isaac Newton initially introduced it in his law of universal gravitation, and Albert Einstein later elaborated on it in his theory of general relativity.
Learn more about Gravitational force at: https://brainly.com/question/27943482
#SPJ1
If 4.44 mol of C,H₁2 reacts with excess O₂, how many moles of CO₂ will be produced by the following combustion reaction?
C₂H2 +80₂6H₂O +5C0₂
moles of CO₂:
mol
Answers
A combustion reaction is a type of chemical reaction that involves the rapid combination of fuel (typically a hydrocarbon) with oxygen, resulting in the release of energy in the form of heat and light.
Combustion reactions are often characterized by the presence of a flame and the production of carbon dioxide (CO₂) and water (H₂O) as products.
In the given balanced combustion reaction:
C₂H₂ + 5O₂ → 4H₂O + 2CO₂
The stoichiometric ratio indicates that 1 mole of C₂H₂ reacts with 2 moles of CO₂ produced. Therefore, if 4.44 moles of C₂H₂ react, we can calculate the moles of CO₂ produced using the ratio:
Moles of CO₂ = (4.44 mol C₂H₂) × (2 mol CO₂ / 1 mol C₂H₂)
Moles of CO₂ = 8.88 mol
Therefore, 8.88 moles of CO₂ will be produced in the combustion reaction.
For more details regarding combustion reaction, visit:
https://brainly.com/question/14335621
#SPJ1
Which is more soluble in water, Calcium Carbonate or Calcium Hydroxide, and why?
Answers
Answer:
Calcium Hydroxide is more soluble in water
Explanation:
Calcium Hydroxide is more soluble in water as all group 2 carbonates are not very soluble while Group 2 hydroxides are moderately soluble.
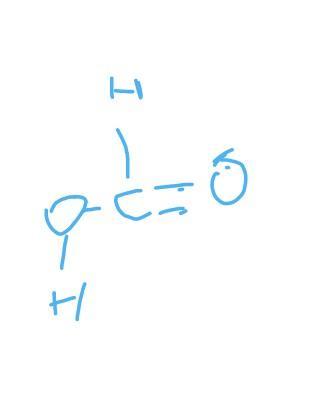
what type of molecule are these and what is the strongest IMFs in between?

Answers
Answer:
CH2O is formaldehyde a covalent compound and its intermolecular forces are week
KCl is an ionic compound formed by electrostatic force of attraction between positive and negative charge. Ionic compounds also exists in three dimensional crystal lattic that is why intermolecular forces in KCl is stronger.
Moreover melting point of KCl is higher than CH2O
Explanation:
How many particles are in 176 grams of Fe(OH)2.
Answers
To solve this we must be knowing each and every concept related to mole and and its calculations. Therefore, 1.17×10²⁴particles are in 176 grams of Fe(OH)\(_2\).
What is mole?A mole is merely a measuring unit. In reality, it is one of International System of Units' seven foundation units (SI). When basically determines are insufficient, new units are created.
Chemical reactions frequently occur at levels that use grams would be inappropriate, but using actual figures of atoms/molecules/ions would also be misleading.
It's much easier to write' mole' than '6.02x10²³' whenever you wish to refer to a huge number of things. That is essentially why and how this particular component was created.
mole =given mass ÷ molar mass
given mass of Fe(OH)\(_2\) =176 grams
Molar mass of Fe(OH)\(_2\) =89.8g/mol
number of moles=176/ 89.8
=1.95moles
number of molecules=1.95moles ×6.022×10²³
=11.7
=1.17×10²⁴particles
Therefore, 1.17×10²⁴particles are in 176 grams of Fe(OH)\(_2\).
To know more about mole, here:
brainly.com/question/15209553
#SPJ1
hedges are used to be courteous in expressing
A. evidence
B. assertions
C. counterclaims
D. critical reading
Answers
Answer:
hedges are used to be courteous in expressing (counterclaims)
A sealed vessel containing sulfur dioxideand nitrogen has a total pressure of 520Torr and partial pressures of 266 Torrnitrogen.What is the partial pressure of sulfurdioxide in Torr?

Answers
They tell us that it is a sealed glass, which means that there is no entry or exit of matter, assuming that the temperature remains constant we can say that the pressure is also constant.
Now, when we have a mixture of gases, the sum of their partial pressures will be equal to the total pressure, therefore we have the following equation:
\(P_A+P_B+P_C\ldots=P_T\)Where PA, PB and PC refer to the partial pressures of gases A, B, and C.
PT is the total pressure.
We have in the question three gases: Carbon Monoxide (CO), Carbon dioxide (CO2), and oxygen (O). The total pressure is 2.68 atm. We can replace the know values into the equation and clear the Partial pressure of carbon.
\(\begin{gathered} P_{CO}+P_{CO2}+P_O=P_T \\ WeclearP_{CO} \\ P_{CO}=P_{T-}P_{CO2}-P_O \\ P_{CO}=2.68\text{atm - 0.65atm-1.56atm} \\ P_{CO}=0.47\text{atm} \end{gathered}\)The partial pressure of carbon monoxide is 0.47 atm
Given the chemical reaction Fe3O4+H2 → Fe + H20, identify the coefficient of H2O in the balanced equation,
A. 0 1
B. 04
C. 06
D. 02
Answers
Answer:
Given the reaction:
Fe+H
2
O→Fe
3
O
4
+H
2
If the number of electrons lost or gained during the changes is x. Then, the value of x is 8.
The balanced chemical equation is as follows:
3Fe+4H
2
O→Fe
3
O
4
+4H
2
The oxidation number of hydrogen changes from +1 (in water) to 0 (in hydrogen molecule).
Thus, net change in the oxidation number of hydrogen per atom is 1.
There are 8 H atoms.
Hence, the total change in the oxidation number of 8 hydrogen atoms is 8.
Hence, 8 electrons are involved in the redox reaction.
Hence, x=8.
A rectangular block has dimension 20m x10mx5m and mass of 400kg calculate the density of the rectangular block

Answers
Answer:
0.4 kg/m^3
Explanation:
v (volume) = 20m x 10m x 5m = 1000 m^3
w (weight) = 400 kg
d (density) = w / v
d = 400 / 1000 = 0.4 kg per cubic meter (kg/m^3)
I apologize if this is a little complex, however all of the math is there, if you're interested then look it over. Otherwise I've provided the answer in the units used for the dimensions and weight provided.
Which of the following particles have the same mass. Proton, Neutron, Electron, None
Answers
Answer: proton and neutron
Explanation:
They both have the mass of 1
4 N2H3CH3 (l) + 5 N2O4 (l) →12 H2O(g) + 9 N2(g) + 4 CO2 (g)The enthalpy of formation for liquid methylhydrazine is +53 kJ/mol and the enthalpy of formation for liquid dinitrogen tetroxide is -20 kJ/mol. Calculate ∆H° for this reaction, ignore significant digits for this question.

Answers
Answer
\(\Delta H_{rxn}\operatorname{\degree}=-4791.6\text{ }kJ\text{/}mol\)Explanation
The given chemical equation for the reaction is:
\(4N_2H_3CH_3(l)+5N_2O_4(l)\text{ }→\text{ }12H_2O\left(g\right)+9N_2\left(g\right)+4CO_2(g)\)From the given table and question, the enthalpies of formation of the reactants ad products are:
\(\begin{gathered} ∆H_f°(N_2H_3CH_{3(l)})=+53\text{ }kJ\text{/}mol \\ \\ ∆H_f°(N_2O_{4(l)})=-20\text{ }kJ\text{/}mol \\ \\ ∆H_f°(H_2O_{(g)})=-258.8\text{ }kJ\text{/}mol \\ \\ ∆H_f°(N_{2(g)})=0\text{ }kJ\text{/}mol \\ \\ ∆H_f°(CO_{2(g)})=-393.5\text{ }kJ\text{/}mol \end{gathered}\)The ∆H° for this reaction can be calculated using the formula below:
\(\Delta H_{rxn}\degree=ΔH_f^{\degree}(products)-ΔH_f^{\degree}(reactants)\)Put the each enthalpy of formation of the reactants and the products into the formula:
\(\begin{gathered} \Delta H_{rxn}\degree=[12(-258.8)+9(0)+4(-393.5)]-[4(+53)+5(-20)] \\ \\ \Delta H_{rxn}\degree=[-3105.6+0-1574]-[212-100] \\ \\ \Delta H_{rxn}\degree=-4679.6-112 \\ \\ \Delta H_{rxn}\degree=-4791.6\text{ }kJ\text{/}mol \end{gathered}\)Therefore, the ∆H° for this reaction is -4791.6 kJ/mol.
How is steel made from the raw product of the blast furnace known
as "pig iron"? What are the advantages of using steel?
List references used (if any were used) to answer this question.
Answers
Steel is produced from pig iron through a process known as steelmaking or iron and steel production.
The pig iron obtained from the blast furnace contains high amounts of carbon, impurities, and other elements. To convert pig iron into steel, the carbon content needs to be reduced to desired levels, and impurities must be removed.One common method of steelmaking is the basic oxygen process (BOP). In this process, pig iron is placed in a vessel called a converter, where oxygen is blown through the molten metal. The oxygen reacts with the carbon and impurities, causing them to oxidize and form gases that are released. Alloying elements and desired additives can be added at this stage to achieve specific steel properties. Another method is the electric arc furnace (EAF), where an electric arc is used to heat and melt the pig iron, allowing impurities to be oxidized and removed.The advantages of using steel are numerous. Steel is strong, durable, and versatile, making it suitable for a wide range of applications. It has high tensile strength, which means it can withstand heavy loads and pressures. Steel is also resistant to corrosion, making it ideal for construction, infrastructure, and transportation projects. It is a recyclable material, contributing to sustainability and reducing environmental impact. Additionally, steel can be fabricated into various shapes and sizes, allowing for customization and flexibility in design.References:
A. Ghosh and A. Chatterjee, Ironmaking and Steelmaking: Theory and Practice, PHI Learning, 2008.
R.H. Tupkary and V.R. Tupkary, An Introduction to Modern Iron Making, Khanna Publishers, 2010.
J.R. Davis, ed., ASM Specialty Handbook: Carbon and Alloy Steels, ASM International, 1995.
for such more questions on production
https://brainly.com/question/25597694
#SPJ8
1.
a) Which is a true statement?
Rocks are made of minerals, but minerals are not rocks.
A mineral is made of two substances, including one organic substance.
A rock is made of only one substance.
Minerals are made of rocks, but rocks are not minerals.
b)
Which type of rock is formed from magma?
sedimentary
metamorphic
igneous
c) .
How is sedimentary rock formed?
It is formed through exposure to weathering and erosion, which breaks it down into sediment. It is then deposited in layers over long periods of time.
It is formed through the cooling of magma and/or lava below or above the surface of Earth.
It is pulled down far beneath the surface of Earth and exposed to extreme temperature and pressure.
d) Recall that color, streak, luster, cleavage, fracture, density, and hardness are the most common properties you can use to distinguish one mineral from another. From the choices below, select the statement that correctly describes cleavage.
how light is reflected on a mineral's surface
how a mineral breaks apart
resistance to scratching
mass per unit volume of a substance
e) Which choice is the parent rock of marble?
granite
shale
sandstone
limestone
Answers
Answer:
i think it would be A
Explanation:
When determining whether the forces acting on an object are balanced or unbalanced, you need to know how much force is
applied to the object. What is force measured in?
ASAP help
Answers
Answer:
Force is measured in Newtons
Explanation:
To find normal force on an incline, use the equation N = mg cos(x), “m” being the object's mass, “g” being the acceleration of gravity, and “x” being the angle of incline. Then, use a calculator to find the cosine of the angle and write down that value.
a chemical compound is a chemical substance composed of many identical molecules (or molecular entities) containing atoms from more than one chemical element held together by chemical bonds. a molecule consisting of atoms of only one element is therefore not a compound. a compound can be transformed into a different substance by a chemical reaction, which may involve interactions with other substances. in this process, bonds between atoms may be broken and/or new bonds formed. there are four major types of compounds, distinguished by how the constituent atoms are bonded together. molecular compounds are held together by covalent bonds; ionic compounds are held together by ionic
Answers
Ionic compounds are held together by ionicbonds. Metallic compounds are held together by metallic bonds, and network covalent compounds are held together by covalent bonds in a continuous network of atoms.
Each type of bond has unique properties that determine the physical and chemical properties of the compound. The type of bond present in a compound determines its reactivity, solubility, and other physical and chemical properties. In summary, a chemical compound is a substance consisting of two or more different elements chemically bonded together, and the properties of a compound depend on the type of bond present in it.
Find more about ionicbonds.
brainly.com/question/15239012
#SPJ4
The picture shows a scientist collecting data from an ice core whats the answer giving brainliest PLEASE HELP

Answers
Answer:
I’m going with her it c
Explanation:
Answer:
it maybe C
Explanation:
How many um are in
22.5 mm?

Answers
Answer:
A
Explanation: That's what I got but I don't know if its correct sorry if it was wrong.
Answer:
the answer is C
Explanation:
Solid,liquid,gas how do they differ in how easily they change shape
Answers
Answer:Solid,Liquid,Gas differ from each other by the following ways:-
Solid:It have defined shape.
Liquid:They have an indefinite shape.
Gas:They have no shape:
Which amphibian organ has a high blood supply and many folds to increase surface area?
a. heart
b. stomach
c. lungs
d. brain
Answers
Answer:
lungs
Explanation:
HỌ5,42
Homework Answered Due Today, 11:59 PM
.
A 5.60E1 g sample of water at 9.910E1 °C is placed in a constant pressure calorimeter. Then, 2.40E1 g of zinc metal at 2.10E1 °C is
added to the water and the temperature drops to 9.70E1 °C. What is the specific heat capacity of the zinc metal measured in this
experiment?
Answers
The specific heat capacity of the zinc metal, given that 2.40×10¹ g of zinc metal at 2.10×10¹ °C is added to the water is 0.27 J/gºC
How do i determine the specific heat capacity of the zinc?First, we shall obtain the heat absorbed by the water when the zinc metal was added. This is shown below:
Mass of water (M) = 5.60×10¹ gInitial temperature (T₁) = 9.910×10¹ °CFinal temperature (T₂) = 9.70×10¹ °CChange in temperature (ΔT) = 9.70×10¹ - 9.910×10¹ = -2.1 °CSpecific heat capacity of water (C) = 4.184 J/gºC Heat absorbed by water (Q) =?Q = MCΔT
= 5.60×10¹ × 4.184 × -2.1
= -492.0384 J
Now, we shall determine the specific heat capacity of the zinc metal. Details below:
Heat absorbed by water (Q) = -492.0384 JHeat released by metal (Q) = 492.0384 JMass of zinc metal (M) = 2.40×10¹ gInitial temperature (T₁) = 2.10×10¹ °CFinal temperature (T₂) = 9.70×10¹ °CChange in temperature (ΔT) = 9.70×10¹ - 2.10×10¹ = 76 °CSpecific heat capacity (C) = ?Q = MCΔT
492.0384 = 2.40×10¹ × C × 76
492.0384 = 1824 × C
Divide both sides by 1824
C = 492.0384 / 1824
= 0.27 J/gºC
Learn more about specific heat capacity:
https://brainly.com/question/19104255
#SPJ1
2.
Which mixture could be a useful buffer in a solution?
acetic acid (CH3CO2H) and hydrochloric acid (HCl)
sodium hydroxide (NaOH) and elemental sodium (Na)
ammonia (NH3) and ammonium chloride (NH4Cl)
acetic acid (CH3CO2H) and ammonia (NH3)
Pls answer quickly
Answers
Ammonia (\(NH_3\)) and ammonium chloride (\(NH_4Cl\)) mixture could be a useful buffer in a solution. Option C
A buffer is a solution that can resist changes in pH when small amounts of acid or base are added. It consists of a weak acid and its conjugate base or a weak base and its conjugate acid. The buffer system works by the principle of Le Chatelier's principle, where the equilibrium is shifted to counteract the changes caused by the addition of an acid or a base.
In option A, acetic acid (\(CH_3CO_2H\)) is a weak acid, but hydrochloric acid (HCl) is a strong acid. This combination does not form a buffer because HCl is completely dissociated in water and cannot provide a significant concentration of its conjugate base.
Option B consists of sodium hydroxide (NaOH), which is a strong base, and elemental sodium (Na), which is a metal. This combination does not form a buffer as there is no weak acid-base pair involved.
Option D contains acetic acid (\(CH_3CO_2H\)), a weak acid, and ammonia (\(NH_3\)), a weak base. Although they are weak acid and base, they do not form a buffer system together as they are both weak acids or bases and lack the required conjugate acid-base pair.
Option C, ammonia (\(NH_3\)), is a weak base, and ammonium chloride (\(NH_4Cl\)) is its conjugate acid. This combination can form a buffer system. When ammonia reacts with water, it forms ammonium ions (NH4+) and hydroxide ions (OH-).
The ammonium ions act as the weak acid, while the ammonia acts as the weak base. The addition of a small amount of acid will be counteracted by the ammonium ions, and the addition of a small amount of base will be counteracted by the ammonia, thus maintaining the pH of the solution relatively stable.
Therefore, option C, consisting of ammonia (\(NH_3\)) and ammonium chloride (\(NH_4Cl\)), is the suitable mixture that could be a useful buffer in a solution.
For more such question on buffer visit:
https://brainly.com/question/13076037
#SPJ8
How many formula units are in 32.4 g of aluminum hydroxide
Answers
Answer:
here are 2.50 x 10^23 formula units in 32.4 g of aluminum hydroxide.
Explanation:
The molar mass of aluminum hydroxide (Al(OH)3) is 78.0 g/mol.
To determine the number of formula units in 32.4 g of aluminum hydroxide, we first need to calculate the number of moles of aluminum hydroxide in 32.4 g:
moles of Al(OH)3 = mass / molar mass
moles of Al(OH)3 = 32.4 g / 78.0 g/mol
moles of Al(OH)3 = 0.415 mol
Next, we use Avogadro's number to convert the number of moles to the number of formula units:
number of formula units = moles of Al(OH)3 x Avogadro's number
number of formula units = 0.415 mol x 6.022 x 10^23/mol
number of formula units = 2.50 x 10^23 formula units
Therefore, there are 2.50 x 10^23 formula units in 32.4 g of aluminum hydroxide.
Which of these casting methods is the most popular, accounting for approx. 70% of all metal casting?
a. Sand casting
b. Shell casting
c. Investment casting
d. Lost foam casting
Answers
Sand casting, which makes up around 70% of all metal casting, is the most common casting technique. A design of the finished object is created in wood or metal and employed in the process of "sand casting,".
Casting techniques are a class of manufacturing procedures used to turn liquid materials like metal, plastic, or ceramics into solid things. The liquid substance is put into a mould and given time to harden and assume the contours of the hollow. Casting techniques come in a variety of forms, including sand casting, investment casting, die casting, shell casting, and lost foam casting. The kinds of materials that can be cast, the degree of accuracy and surface quality, and the complexity of the pieces that can be created are only a few of the distinctive characteristics that each casting technique has. The automobile, aerospace, and construction industries all use casting techniques extensively.
Learn more about casting methods here:
https://brainly.com/question/15359594
#SPJ4
A reaction produces 0.831 moles of H2O . How many molecules of H2O are produced?
Answers
Please and thank you
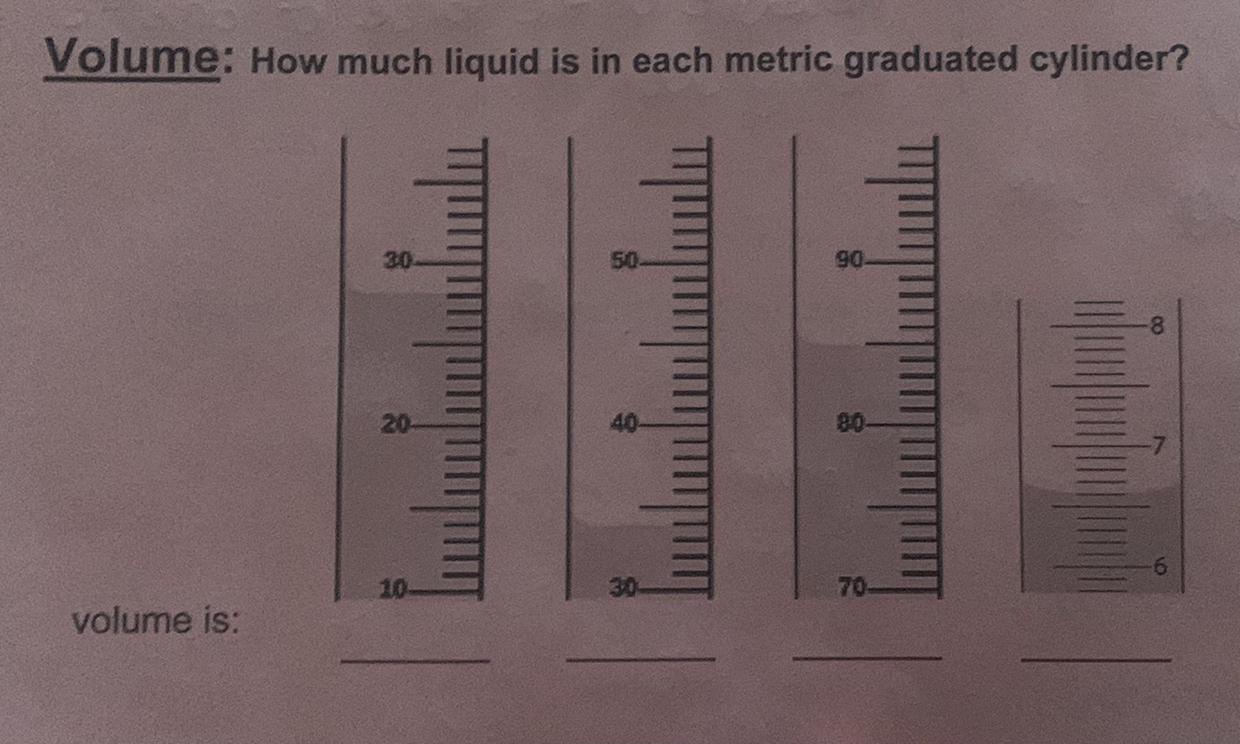
Answers
Answer:28, 34, 85, 6.6
Explanation:
How many molecules of glucose (C6H12O6,M=180.18g/mol) are contained in 144mL of 0.280 M glucose solution?
Answers
Volume of Glucose solution = 144 mL OR 0.144 L
Molarity of Glucose solution = 0.280 M
Number of moles of Glucose in the solution:From the definition of Molarity, we know that:
Molarity = number of moles / volume(in L)
in this equation, multiplying both sides by Volume, we get:
number of moles = Molarity*Volume(in L)
replacing the variables
Number of moles = 0.280*(0.144L)
Number of moles = 0.04
Number of Molecules in the solution:
We know that:
Number of molecules = Number of Moles * Avogadro's number
Number of molecules = 0.04 * 6.022 * 10²³
Number of molecules = 0.24088 * 10²³
Number of molecules = 2.4088 * 10²²
Therefore, there are 2.4088 * 10²² molecules of Glucose in the given solution
how many atoms in 0.034 moles of titanium
Answers
Answer:
\( \huge{ \boxed{2.0468 \times {10}^{22} \: \text{atoms} }}\)
Explanation:
To find the number of entities in a given substance we use the formula
\( \bold{N = n \times L} \)
where n is the number of moles
N is the number of entities
L is the Avogadro's constant which is
6.02 × 10²³ entities
From the question
n = 0.034 moles
N = 0.034 × 6.02 × 10²³ = 2.0468 × 10²²
We have the final answer as
\( \bold{2.0468 \times {10}^{22} \: \text{atoms}}\)