Answers
According to chemical equilibrium , if temperature and pressure increases the concentration of CO₂ increases and if water is removed and oxygen is added the concentration increases and decreases respectively.
Chemical equilibrium is defined as the condition which arises during the course of a reversible chemical reaction with no net change in amount of reactants and products.A reversible chemical reaction is the one wherein the products as soon as they are formed react together to produce back the reactants.
Factors which affect chemical equilibrium are change in concentration , change in pressure and temperature and presence of catalyst.
Learn more about chemical equilibrium,here:
https://brainly.com/question/3920294
#SPJ1
Related Questions
If the theoretical yield of a reaction is 332.5 g and the percent yield for the reaction is 38 percent, what's the actual yield of product in grams?
A)
126.4 g
B)
8.74 g
C)
116.3 g
D)
12616 g
Answers
Discuss the following statement:
"Small changes in the chemical nature of polysaccharides results in significant differences in biological function"
Answers
Answer:
Explanation:
Small changes in the chemical nature of polysaccharides can make a big difference in how they work in our bodies. Polysaccharides are complex carbohydrates found in things like fiber and medicines. Even tiny changes in their structure can affect how they are digested, how they interact with cells, and their overall impact on our health. Scientists can use these changes to create materials with specific properties or develop new treatments. So, even small tweaks in polysaccharides can have a significant impact on how they function in our bodies.
Calculate the moles of HCL added to the acidic buffer before its buffering capacity was exceeded.
HCl Volume:pH
5ml:3.84
10ml:2.41
15ml:1.8
20ml:1.71
Answers
Compute the initial moles of HCl present in the buffer before any HCl was added. This may be accomplished by utilising the buffer's starting pH and the known dissociation constant (Ka) for the weak acid in the buffer. For example, if the buffer is formed of acetic acid (CH3COOH) and its starting pH is 4.5, we may determine the initial concentration of CH3COOH in the buffer using the Ka value for acetic acid (1.8 x 10-5).
Subtract the total moles of H+ at the location from the beginning moles of H+ in the buffer.
What exactly is an acid?In a chemical process, an acid either provides protons (hydrogen ions) or receives electrons. Acids have a pH value less than 7 and are distinguished by their sour taste and capacity to dissolve certain metals, such as zinc and iron
To know more about acid visit :
brainly.com/question/29796621
#SPJ1
What physical property of matter can be measured using the triple beam balance?
A
volume
B
mass
C
height
D
length
SUBMIT ANSWER
Answers
Answer:
B) Mass- I searched it up
Steel is an alloy of iron and carbon. The addition of carbon to iron enhances which of the following properties of iron metal?
O A hardness
O B. malleability
O C. ductility
O D. softness
Answers
Answer:
The answer should be A. Adding carbon to iron makes it tougher and stronger.
The addition of carbon atom to iron metal, enhances the property of hardness of metal.
What is alloy?Alloy is a compound which is formed by the mixture of two or more than two metals with different properties to make a new compound with better properties.
When we add carbon atom in the iron metal, it deviates the crystal lattice property of iron and makes it more harder. So, the content of carbon in the iron is directly proportional to the hardness of iron metal.
Hence, option (A) is correct i.e. hardness.
To know more about hardness, visit the below link:
https://brainly.com/question/18173707
a 0.03 mol sample of c3h8 is reacted with just enough o2 to use up both reactants in a 1 l flask at 300 k. the total pressure in the flask after the reaction is complete is closest to which of the following?
Answers
Answer:
A) 5.0 atm
Explanation:
You want to know the pressure in a 1 L flask at 300 K when 0.03 mol of C₃H₈ reacts completely with O₂.
ReactionWe suppose the reaction of interest is ...
C₃H₈(g) +5O₂(g) ⇒ 3CO₂(g) +4H₂O(g)
This shows us there are 3+4 = 7 moles of products for each mole of C₃H₈ in the reaction. That is, there will be 0.03 mole × 7 = 0.21 mole of products.
Gas lawThe gas law equation tells us the final pressure can be computed by ...
PV = nRT
P = nRT/V = (0.21 mol)(0.082 L·atm/(mol·K)(300 K)/(1 L) ≈ 5.166 atm
The total pressure in the flask after the reaction is closest to ...
A) 5.0 atm

The temperature of 100 grams of water changes from 16°C to 20°C. What is the total number of calories of heat energy absorbed by the water?
A.
25
B.
40
C.
100
D.
400
Answers
Answer:
D. 400 cal
Explanation:
Spec heat of water 1 cal/gm-C
1 cal / gm-C * 100 gm * ( 20-16 C) = 400 cal
17. HAZWOPER training and certification recognizes:
a. A large number (as much as 80%) will self-present or be self-referred victims
b. Awareness level training will promote proper initial triage actions
c.
Victims will use any entrance they can enter at the hospital, in addition to the
emergency department entrance
d. Both A and C
Answers
HAZWOPER training and certification recognize:
a large number (as much as 80%) will self-present or be self-referred victimsVictims will use any entrance they can enter at the hospital, in addition to the emergency department entranceThe correct option is both A and C
What is the HAZWOPER training and certification?HAZWOPER (Hazardous Waste Operations and Emergency Response) training and certification recognize that a large number of victims (as much as 80%) in hazardous waste incidents or emergencies will self-present or be self-referred for medical treatment.
Additionally, HAZWOPER training acknowledges that victims may use any entrance they can access at a hospital, not just the emergency department entrance.
This is because individuals affected by hazardous materials may arrive at different areas of the hospital seeking medical assistance.
Therefore, option d. Both A and C are correct statements regarding the recognition of HAZWOPER training and certification.
Learn more about HAZWOPER at: https://brainly.com/question/31561828
#SPJ1
Experiment 2: Copper(II) carbonate hydroxide produces one mole of water (=18.015 g/mol) for every two moles of solid product (=79.545 g/mol) produced. If 3.597 g of solid product were produced during the reaction, how many grams of water were realeased as water vapor?
Answers
Which compound has a functional group that contains two oxygen atoms?
Answers
The compound that has a functional group containing two oxygen atoms is peroxide. In addition to peroxides, there are other compounds that contain functional groups with two oxygen atoms. These include carboxylic acids and esters.
Peroxide compounds, such as hydrogen peroxide (H₂O₂) or organic peroxides, have a functional group (-O-O-) where two oxygen atoms are bonded together. This group is responsible for the characteristic properties and reactivity of peroxides.
Carboxylic acids: Carboxylic acids have the functional group -COOH, which consists of a carbonyl group (C=O) and a hydroxyl group (-OH) bonded to the same carbon atom. While carboxylic acids have one oxygen atom from the carbonyl group, the hydroxyl group provides the second oxygen atom.
Esters: Esters have the functional group -COO-, which consists of a carbonyl group (C=O) and an oxygen atom (-O-) bonded to the same carbon atom. This arrangement gives esters two oxygen atoms within their functional group.
Hence, the compounds with functional groups containing two oxygen atoms include peroxides, carboxylic acids, and esters.
Learn more about functional groups here:
https://brainly.com/question/28563874
#SPJ 2
Can someone help me and explain why they got what they got?

Answers
Answer:
24.32 amu
Explanation:
From the question given above, the following data were obtained:
Isotope A (Mg–24):
Mass of A = 24 amu
Abundance (A%) = 79%
Isotope B (Mg–25):
Mass of B = 25 amu
Abundance (B%) = 10%
Isotope C (Mg–26):
Mass of C = 26 amu
Abundance (C%) = 11%
Average atomic mass of Mg =?
Average atomic mass = [(Mass of A × A%)/100] + [(Mass of B × B%)/100] + [(Mass of C × C%)/100]
= [(24 × 79)/100] + [(25 × 10)/100] + [(26 × 11)/100]
= 18.96 + 2.5 + 2.86
= 24.32 amu
Thus, the average atomic mass of Mg is 24.32 amu
A cell must work to maintain a stable internal environment. It is also important for the environment around the cell to be stable. What reasoning explains what happens when the concentration of water inside a cell is lower than the concentration of water outside the cell?
The cell loses nutrients.
The cell splits and creates two new cells.
The cell gains too many lipids and carbohydrates.
The cell either loses water and dries up or gains too much water and bursts.
(science 7th grade)
Answers
Answer: the last one
Explanation: if a cell has too little water, it will begin to function incorrectly, and if a cell has too much water it will burst.
How do I do this? What are the answers to the 5 questions shown?

Answers
Answer:
1. C₃H₆O₃
2. C₆H₁₂
3. C₆H₂₄O₆
4. C₆H₆
5. N₂O₄
Explanation:
1. Determination of the molecular formula.
Empirical formula => CH₂O
Mass of compound = 90 g
Molecular formula =?
Molecular formula = n × Empirical formula = mass of compound
[CH₂O]ₙ = 90
[12 + (2×1) + 16]n = 90
[12 + 2 + 16]n = 90
30n = 90
Divide both side by 30
n = 90/30
n = 3
Molecular formula = [CH₂O]ₙ
Molecular formula = [CH₂O]₃
Molecular formula = C₃H₆O₃
2. Determination of the molecular formula.
Empirical formula => CH₂
Mass of compound = 84 g
Molecular formula =?
Molecular formula = n × Empirical formula = mass of compound
[CH₂]ₙ = 84
[12 + (2×1)]n = 84
[12 + 2]n = 84
14n = 84
Divide both side by 14
n = 84/14
n = 6
Molecular formula = [CH₂]ₙ
Molecular formula = [CH₂]₆
Molecular formula = C₆H₁₂
3. Determination of the molecular formula.
Empirical formula => CH₄O
Mass of compound = 192 g
Molecular formula =?
Molecular formula = n × Empirical formula = mass of compound
[CH₄O]ₙ = 192
[12 + (4×1) + 16]n = 192
[12 + 4 + 16]n = 192
32n = 192
Divide both side by 32
n = 192/32
n = 6
Molecular formula = [CH₄O]ₙ
Molecular formula = [CH₄O]₆
Molecular formula = C₆H₂₄O₆
4. Determination of the molecular formula.
Empirical formula => CH
Mass of compound = 78 g
Molecular formula =?
Molecular formula = n × Empirical formula = mass of compound
[CH]ₙ = 78
[12 + 1]n = 78
13n = 78
Divide both side by 13
n = 78/13
n = 6
Molecular formula = [CH]ₙ
Molecular formula = [CH]₆
Molecular formula = C₆H₆
5. Determination of the molecular formula.
Empirical formula => NO₂
Mass of compound = 92 g
Molecular formula =?
Molecular formula = n × Empirical formula = mass of compound
[NO₂]ₙ = 92
[14 + (2×16)]n = 92
[14 + 32]n = 92
46n = 92
Divide both side by 46
n = 92/46
n = 2
Molecular formula = [NO₂]ₙ
Molecular formula = [NO₂]₂
Molecular formula = N₂O₄
Which reagent is the limiting reactant when 0.400 mol Al(OH)3 and 0.400 mol H2SO4 are allowed to react
Answers
Answer:
H₂SO₄ will be the limiting reagent.
Explanation:
The balanced reaction is:
2 Al(OH)₃ + 3 H₂SO₄ → Al₂(SO₄)₃ + 6 H₂O
The limiting reagent is one that is consumed first in its entirety, determining the amount of product in the reaction. When the limiting reagent is finished, the chemical reaction will stop.
To determine the limiting reagent, it is possible to use the reaction stoichiometry of the reaction (that is, the relationship between the amount of reagents and products in a chemical reaction).
You can use a simple rule of three as follows: if by stoichiometry 2 moles of Al(OH)₃ reacts with 3 moles of H₂SO₄, how much moles of H₂SO₄ will be needed if 0.4 moles of Al(OH)₃ react?
\(moles of H_{2} SO_{4} =\frac{0.4 moles of Al(OH)_{3}*3 moles of H_{2} SO_{4} }{2 moles of Al(OH)_{3}}\)
moles of H₂SO₄= 0.6 moles
But 0.6 moles of H₂SO₄ are not available, 0.40 moles are available. Since you have less moles than you need to react with 0.4 moles of Al(OH)₃, H₂SO₄ will be the limiting reagent.
Balance and Classify the following chemical reactions:.(NH4)2S (aq) + ZnCl2 (aq) ® ZnS (s) + NH4Cl (aq)
Answers
In order to properly balance an equation, we need to make sure that the same amount of elements on the reactants side matches the number of elements on the products side, we can do that by increasing the number in front of each molecule, the so called stoichiometric coefficient. In the reaction from the question we can properly balance by adding the following stoichiometric coefficients
(NH4)2S + ZnCl2 -> ZnS + 2 NH4Cl
Since we have Zn and NH4 switching places and forming new compounds, we have a perfect example of a Douple Displacement reaction
Approximately 50% of our bone is chemically calcium phosphate, Ca3(PO4)2
If an adult has 12 kg of bone, calculate the mass of calcium is present
Answers
Define biotechnology. } List two advantages in the use of biotechnology
Answers
Advantages of biotechnology:
Improvement of plants and animal breeds to give a high yield of their products.
Pests and pathogen control in agriculture which will reduce the loss of yield in food crops.
Synthesis of biocatalyst which can be used for enhancing the reactions which can be carried out in vitro or laboratory conditions.
Sewage treatment or water recycling can be done with the help of transgenic microbes which have better efficiency and speed.
Biotechnology is the use of living organisms or other biological systems in the manufacture of drugs or other products or for environmental management, as in waste recycling: includes the use of bioreactors in manufacturing, microorganisms to degrade oil slicks or organic waste, genetically engineered bacteria to produce human hormones, and monoclonal antibodies to identify antigens.
Biotech offers the possibility of improving human health, the environment, and agriculture while creating more sustainable modes of production.
Determine the empirical formula of Magnesium Oxide from following data. Type the calculations.
Mass of Crucible and Cover + magnesium ribbon (before heating) =27.60 g
Mass of crucible and Cover = 27.30 g
Mass of magnesium metal =??
Mass of crucible and cover + magnesium oxide (after heating) = 27.80 g
Mass of combined oxide (after heating - before heating) =??
Answers
mass of combined oxide = ( mass of crucible and cover + magnesium oxide) - (mass of crucible and cover + magnesium oxide) - (magnesium metal )
emprical formula :
find no of mole of Magnesium
= mass divide atomic number
= 0.0125
find for oxygen also
= 0.0125
ratio
0.0125 : 0.0125
1:1
MgO
HELP!!
What structure is found in the nucleus of a cell and is made up of coiled strands of DNA?
O protein
O gene
O chromosome
O centriole
Answers
NEED ASAP!
What is the resolution of a monochromator, Δλeff, with a exit slit width of 500 micrometers and a \(D^{-1}\) of 1.8 nm/mm? Express the answer in nm.
Answers
The resolution of the monochromator is 3.6nm
What is a MonochromatorA monochromator is an optical device that is used to isolate a specific wavelength or range of wavelengths of light from a broader spectrum of light. It is typically used in spectroscopy, where the goal is to measure the intensity of light at a specific wavelength or over a range of wavelengths.
The resolution of a monochromator, Δλeff, is given by the equation: Δλeff = (D^-1) * (exit slit width)
Plugging in the given values:
Δλeff = (1.8 nm/mm) * (500 micrometers)
Converting micrometers to millimeters:
Δλeff = (1.8 nm/mm) * (0.5 mm)
Δλeff = 3.6 nm
Learn more on monochromator here;
https://brainly.com/question/917245
#SPJ1
A piece of metal is heated to 250 degrees then placed in a bucket of water with an initial temperature of 15 degrees. After 10 minutes, the system reaches a point of thermal equilibrium. The water is now 22 degrees. Which of the following is the most likely temperature of the piece of metal?
Answers
Answer: 22 degrees
Explanation:
Most of the information in this question is put there to throw you off- the only important piece of information here is the final temperature of the water.
In a system of say 2, like in this example, whichever object has more energy in the form of heat will transfer that heat to the object with lesser heat if there is an available path to transfer heat between them. Once the two objects are at the same temperature, however, heat can not be transferred from one to another since neither object has more heat than the other to transfer- this is thermal equilibrium.
This question tells us that the system is at thermal equilibrium- when both objects are at the same temperature. Given the temperature of one object, the water, we then know the other object, the metal is the same temperature.
The Universal Space Agency wants to know the results of your Sim investigation. Write your report to the lead chemist, explaining why drops of liquid water appeared on the outside of the cold, but not the warm, soda can. Use evidence from your investigation in your explanation.
Answers
Answer:
due to warm temperature
Explanation:
Explanation:
There is water present in liquid form instead of liquid nitrogen on the outer side of the can because of the temperature present in the surrounding environment. The temperature around the can is little warm which is favourable for the water but not for the liquid nitrogen. The liquid nitrogen present in liquid state when the temperature of the surrounding is too cold. if it is warm, the liquid nitrogen starts boiling and converts into gaseous state so that's why the warmer temperature is responsible for the presence of water not the liquid nitrogen.
871g of sodium chloride is how many moles
Answers
Answer:
14.9 mol
Explanation:
To find the number of moles in a given mass of a sample of sodium chloride (NaCl), we can multiply the number of grams in the sample by the molar mass of sodium chloride, which is 58.44 g/mol.
871 g × (1 mol / 58.44 g)
= 871/58.44 mol
≈ 14.9 mol
Note that we rounded to 3 significant figures in the final answer because that is how many significant figures were given in the mass measurement of the sodium chloride sample.
Descriptions of enzyme mechanisms often contain the terms intermediate, transition state, and transition state analogue. The terms intermediate and transition state both describe chemical species along the reaction pathway, but there are important distinctions between the two. Transition state analogues are often used in enzyme mechanism studies. Match each phrase to the term that it describes.
a. Compound designed to mimic the structure of a transition state
b. Contains partially formed and partially broken bonds
d. Often serves as a potent enzyme inhibior
Answers
Solution :
The intermediate state and the transition state are used to describe the chemical species that is along the reaction pathway.
Therefore, the phrase of the term that it describes each of the terms, intermediate, transition state analogue and transition state are as follows :
a. Compound which is designed to mimic the structure of the transition state
-- transition state analogue
b. Containing partially formed as well as partially broken bonds.
-- transition state
d. Often serves as the potent enzyme inhibitor
-- transition state analogue
A physician orders 3 L of 35% irrigation solution to be prepared from a mixture of 12% and 44% solutions. How many mL of 44% solution are needed?
Answers
2156.25 mL of the 44% solution are needed. A homogeneous mixture has a uniform composition throughout and is also called a solution.
What is Mixture?
A mixture is a combination of two or more substances that are physically combined but not chemically bonded to each other. In a mixture, each substance retains its own chemical identity and properties, and can be separated from the mixture using physical means, such as filtration, evaporation, or distillation. Mixtures can be homogeneous or heterogeneous.
Let x be the volume (in mL) of the 44% solution needed.
Since we want to end up with 3 L (or 3000 mL) of 35% solution, we can set up the following equation to solve for x:
0.12(3000 - x) + 0.44x = 0.35(3000)
This equation balances the amount of solute (i.e. the active ingredient) in the mixture before and after mixing.
Simplifying and solving for x, we get:
360 - 0.12x + 0.44x = 1050
0.32x = 690
x = 2156.25 mL (rounded to the nearest hundredth)
Therefore, 2156.25 mL of the 44% solution are needed.
Learn more about Mixture from given link
https://brainly.com/question/2331419
#SPJ1
What the answer please help

Answers
Answer:
cell,tissue ,organs,organism ,organ system
Answer:
cells, tissue, organ, organ system , and organism
Explanation:
im confused help me please
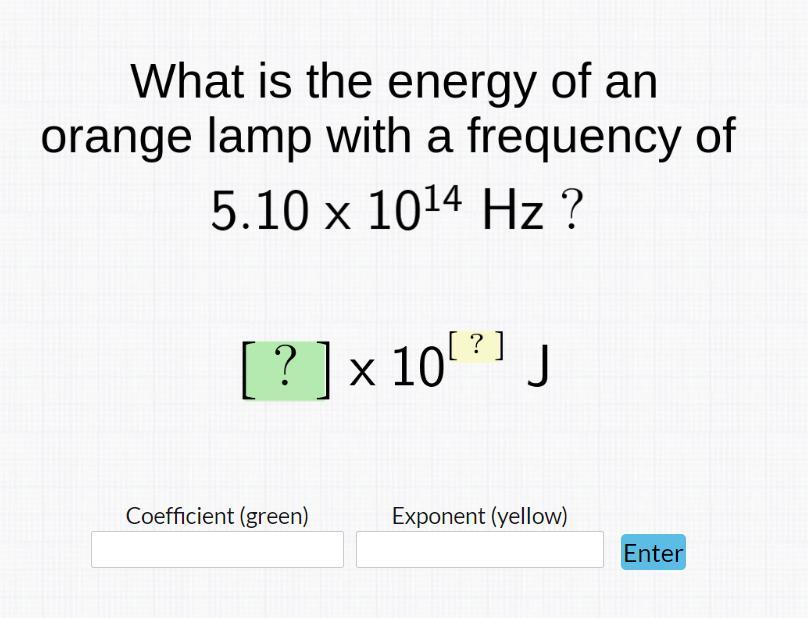
Answers
The energy of an orange lamp with a frequency of 5.10 x 10¹⁴ Hz is 3.38 x 10⁻¹⁹ J.
What is frequency ?Frequency is defined as the number occurrences of repeated events per unit of time. SI unit is hertz (Hz).
Briefing:Using the formula,
E = hν
where,
E = energy in joules (J)
h = Planck's constant = 6.63 x 10⁻³⁴ J
ν = frequency in hertz
Given :
Frequency = 5.10 x 10¹⁴ Hz
h = 6.63 x 10⁻³⁴ J
Putting the values,
E = (6.63 x 10⁻³⁴) x (5.10 x 10¹⁴) J
E = 3.38 x 10⁻¹⁹ J
The energy of an orange lamp with a frequency of 5.10 x 10¹⁴ is 3.38 x 10⁻¹⁹J.
To know more about Frequency visit:
https://brainly.com/question/14316711
#SPJ13
The complete question is -
What is the energy of an orange lamp with a frequency of 5.10 x 10¹⁴ Hz ?
Who was the first inventor of electric bulb?
a) Edison
b) Joseph
c) Maxim
Answers
Thomas Edison is the answer...no a
The esterification reaction is a name reaction in organic chemistry, where it is called ____________ esterification. In addition, when the reverse reaction is promoted by using a base, it is called a ____________ reaction.
Answers
Answer:
rank me as brainliest
Explanation:
The esterification reaction is a name reaction in organic chemistry, where it is called Fischer esterification. In addition, when the reverse reaction is promoted by using a base, it is called a saponification reaction.
Answer:
The esterification reaction is a name reaction in organic chemistry, where it is called Fischer esterification. In addition, when the reverse reaction is promoted by using a base, it is called a hydrolysis reaction.
Explanation:
The Fischer esterification is a type of organic reaction that involves the conversion of a carboxylic acid and an alcohol into an ester and water, catalyzed by an acid catalyst. This reaction is named after its discoverer, Emil Fischer, a German chemist who first described the reaction in 1895.
The general equation for Fischer esterification is:
Carboxylic acid + Alcohol → Ester + Water
For example, the reaction between acetic acid and ethanol to form ethyl acetate (a commonly used solvent and flavoring agent) can be represented as follows:
CH3COOH + C2H5OH → CH3COOC2H5 + H2O
The Fischer esterification is an important reaction in organic chemistry because esters are important compounds in a variety of applications, including the food industry (as flavorings and fragrances), the pharmaceutical industry (as intermediates in the synthesis of drugs), and the polymer industry (as monomers).
When the reverse reaction of Fischer esterification is promoted by using a base, it is called a hydrolysis reaction. Hydrolysis is the process of breaking down a compound by adding water. In the case of esters, hydrolysis occurs when the ester bond is broken by the addition of water, yielding a carboxylic acid and an alcohol.
The general equation for hydrolysis of an ester is:
Ester + Water → Carboxylic acid + Alcohol
For example, the hydrolysis of ethyl acetate can be represented as follows:
CH3COOC2H5 + H2O → CH3COOH + C2H5OH
Hydrolysis of esters is an important reaction in organic chemistry because it is a common route for the degradation of esters in nature, as well as in many industrial processes where it is used for the production of carboxylic acids and alcohols.
Figure1 of 1
A diagram shows that over a distance of 1.6 times 10 to negative 7 meters, wave A completes 4 and a half cycles and wave B completes two cycles.
Part A
What is the wavelength of wave A?
Express your answer using two significant figures.
λ =_________m
Part B
What is the wavelength of wave B?
λ =_________m
Answers
Wavelength is a physical quantity that measures the distance between two consecutive points in a wave that are in phase, or have the same displacement and direction of motion.
What is Wavelength?
The distance separating two wave peaks or troughs is known as the wavelength. It is typically denoted by the Greek letter lambda (λ) and is measured in units of length, such as meters (m) or nanometers (nm).
Part A:
To find the wavelength of wave A, we can use the equation:
wavelength = distance / number of cycles
Plugging in the given values, we get:
wavelength = (1.6 x 10⁻⁷m) / 4.5 cycles
wavelength = 3.56 x 10⁻⁸ m
Rounding to two significant figures, we get:
wavelength = 3.6 x 10⁻⁸ m
Part B:
To find the wavelength of wave B, we can use the same equation:
wavelength = distance / number of cycles
Plugging in the given values, we get:
wavelength = (1.6 x 10⁻⁷ m) / 2 cycles
wavelength =\(8.0 \times 10^{-8 }m\)
Rounding to two significant figures, we get:
wavelength = 8.0 x 10⁻⁸ m
Learn more about Wavelength from given link
https://brainly.com/question/10750459
#SPJ1
