Answers
Answer:
E = 5×10⁻¹⁹ J
Explanation:
Given data:
Wavelength = 400 nm (400×10⁻⁹ m)
Energy of wave = ?
Solution:
Formula:
E = h c/λ
c = 3×10⁸ m/s
h = 6.63×10⁻³⁴ Js
Now we will put the values in formula.
E = 6.63×10⁻³⁴ Js × 3×10⁸ m/s /400×10⁻⁹ m
E = 19.89×10⁻²⁶ J.m / 400×10⁻⁹ m
E = 0.05×10⁻¹⁷ J
E = 5×10⁻¹⁹ J
Related Questions
Given Kc = 2367 at 999 K, calculate Kp for the reaction at equilibrium: CS₂(g) + 3Cl₂(g) → S₂Cl3(g) + CCl4(8) R = 0.08206 L atm K-¹ mol-¹
Answers
The value of Kp for the given reaction at equilibrium is approximately 192,986.689 L atm mol⁻¹.
To calculate the equilibrium constant Kp for the given reaction, we can use the relationship between Kc and Kp, which is expressed as:
Kp = Kc * (RT)^Δn
Where:
- Kp is the equilibrium constant in terms of partial pressures.
- Kc is the equilibrium constant in terms of concentrations.
- R is the ideal gas constant (0.08206 L atm K⁻¹ mol⁻¹).
- T is the temperature in Kelvin.
- Δn is the change in the number of moles of gas (sum of products - sum of reactants).
In this case, the reaction involves four moles of gas on the left-hand side (reactants) and five moles of gas on the right-hand side (products). Therefore, Δn = 5 - 4 = 1.
Given that Kc = 2367 at 999 K, we can substitute these values into the equation:
Kp = 2367 * (0.08206 L atm K⁻¹ mol⁻¹ * 999 K)^1
Simplifying the expression:
Kp = 2367 * (81.367 L atm mol⁻¹)
Calculating the product:
Kp ≈ 192,986.689 L atm mol⁻¹
Therefore, the value of Kp for the given reaction at equilibrium is approximately 192,986.689 L atm mol⁻¹.
For more questions on equilibrium, click on:
https://brainly.com/question/517289
#SPJ8
If carbon can count the shared hydrogen
atoms for itself, how many electrons are
now in carbon's outer orbital?
Answers
Answer:
Mars and Orbital code saber and the alien
Two students prepared aqueous solutions of LiCl and measured the properties, as shown in the table above. Both students observed that the solid LiCl readily dissolved in H2O. The students drew particle diagrams to explain the changes in the enthalpy and entropy of dissolution for LiCl based on their results and observations. Based on this information, the better particle diagram was drawn by which student, and why is that diagram more accurate?
A) The better particle diagram was drawn by Student 1 because when LiCl dissolves in water, it dissociates into Li+ and Cl− ions causing an increase in entropy.
B) The better particle diagram was drawn by Student 1 because when LiCl dissolves in water, it dissociates into Li+ and Cl− ions indicating that the dissolution of LiCl is exothermic.
C) The better particle diagram was drawn by Student 2 because it shows that LiCl does not dissociate, resulting in a decrease in entropy for the dissolution of LiCl.
D) The better particle diagram was drawn by Student 2 because it shows that LiCl does not dissociate, resulting in an endothermic process for the dissolution of LiCl.
Answers
Answer:
Solubility
Explanation:
Solid LiCl dissolves in water because:
A. The Li+ ions are attracted to the oxygen atom (partially -ve charge) of water.
D. The Cl– ions are attracted to the hydrogen atom (partially +ve charge) of water.
The better particle diagram was drawn by Student 1 because when LiCl dissolves in water, it dissociates into Li+ and Cl− ions causing an increase in entropy. The correct option is option A.
What is solubility?The maximum amount of a material that may dissolve in another is known as its solubility. A saturated solution is created when a solvent can dissolve its maximum quantity of solute before reaching equilibrium.
A supersaturated solution results when extra solutes are dissolved past the equilibrium solubility limit under specific circumstances. More solute cannot raise the concentration in the solution past saturation or supersaturation. Instead, the extra solute begins to separate from the solution as a precipitate. The better particle diagram was drawn by Student 1 because when LiCl dissolves in water, it dissociates into Li+ and Cl− ions causing an increase in entropy.
Therefore, the correct option is option A.
To learn more about solubility, here:
https://brainly.com/question/14366471
#SPJ6
what are the impact of soil Science to the development of Ghana's agriculture?
Answers
In an ecosystem, the impact of soil Science to the development of Ghana's agriculture is that it provides suitable conditions for root germination and growth.
What is an ecosystem?Ecosystem is defined as a system which consists of all living organisms and the physical components with which the living beings interact. The abiotic and biotic components are linked to each other through nutrient cycles and flow of energy.
Energy enters the system through the process of photosynthesis .Animals play an important role in transfer of energy as they feed on each other.As a result of this transfer of matter and energy takes place through the system .Living organisms also influence the quantity of biomass present.By decomposition of dead plants and animals by microbes nutrients are released back in to the soil.
Learn more about ecosystem,here:
https://brainly.com/question/1673533
#SPJ9
How many oxygen atoms will there be in the balanced equation of Al+O2-Al2O3
Answers
Answer: 10
Explanation:
4Al + 2O2 --> 2Al2O3
This is the balanced equation so there are 2*2 +2*3 = 4+6 =10
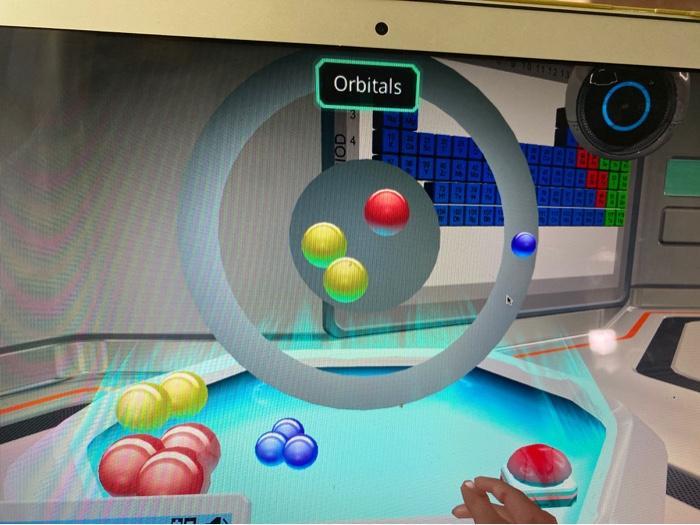
Create a cation. Click the red button once
you are finished.
Answers
In order to create a cation, one first needs to understand that a cation is an atom with excessive positive particles (proton) as compared to negative particles (electrons). Hence, a cation is always positively charge.
From the image simulating the orbital, proton and neutron are represented by either of the red or yellow ball respectively since both particles occupy the innermost part of the atom. The electron is usually revolves in the orbitals and hence, is represented by the blue ball.
Assuming that the proton is represented by the red ball, all that is required to create a cation would be to have more red ball in the nucleus than blue balls outside the nucleus.
More on the charged atoms can be found here: https://brainly.com/question/9264485
A bowling ball and basketball collide, what direction do they go and how is energy transferred?
Answers
Answer:
When the batter hits the ball, there is a force applied, and energy is transferred. The ball will move in the direction the force is pushing it. If two objects collide, energy will be transferred between both, and there will be a change in motion.
Explanation:
which is the graph of the function g(x) = f(-x)
Answers
To graph the function g(x) = f(-x), you can start with the graph of f(x) and then reflect it about the y-axis.
What is a graph of the function g(x) = f(-x)?To find the graph of the function g(x) = f(-x), we can start with the graph of the function f(x) and then reflect it about the y-axis.
If the graph of f(x) is symmetric with respect to the y-axis, meaning it is unchanged when reflected, then g(x) = f(-x) will have the same graph as f(x).
However, if the graph of f(x) is not symmetric with respect to the y-axis, then g(x) = f(-x) will be a reflection of f(x) about the y-axis.
In either case, the resulting graph of g(x) = f(-x) will be symmetric with respect to the y-axis.
Learn more about the graph of functions at: https://brainly.com/question/17089414
#SPJ1

Is it correct ?!???!);$);

Answers
Answer:
None of the above
Explanation:
process of elimination i guess, also delta E measures the amount of change in visual perception of two colors, so it probably wont mean w (work).
then again i may be wrong-
Milk of magnesia, which is an aqueous suspension of magnesium hydroxide, is used as an antacid in the reaction below. How many molecules of HCl would have to be present to form 34.52 g of MgCl₂?
Mg(OH)₂(s) + 2 HCl(aq) → 2 H₂O(l) + MgCl₂(aq)
Answers
Approximately 4.37 x 10^23 molecules of HCl would be required to form 34.52 g of MgCl₂.
To determine the number of molecules of HCl required to form 34.52 g of MgCl₂, we need to use the molar mass and stoichiometry of the balanced equation:
Mg(OH)₂(s) + 2 HCl(aq) → 2 H₂O(l) + MgCl₂(aq)
The molar mass of MgCl₂ is 95.21 g/mol.
First, we need to calculate the number of moles of MgCl₂ formed:
Moles of MgCl₂ = mass of MgCl₂ / molar mass of MgCl₂
Moles of MgCl₂ = 34.52 g / 95.21 g/mol
Moles of MgCl₂ = 0.363 mol
According to the balanced equation, the stoichiometric ratio between HCl and MgCl₂ is 2:1. Therefore, the moles of HCl required can be calculated as follows:
Moles of HCl = 2 * Moles of MgCl₂
Moles of HCl = 2 * 0.363 mol
Moles of HCl = 0.726 mol
To calculate the number of molecules, we need to use Avogadro's number, which is approximately 6.022 x 10^23 molecules/mol.
Number of molecules of HCl = Moles of HCl * Avogadro's number
Number of molecules of HCl = 0.726 mol * 6.022 x 10^23 molecules/mol
Number of molecules of HCl = 4.37 x 10^23 molecules
Therefore, approximately 4.37 x 10^23 molecules of HCl would be required to form 34.52 g of MgCl₂.
For more such questions on molecules
https://brainly.com/question/1351818
#SPJ8
consider a gaseous reaction at equilibrium in a container with a moving piston. if the piston is moved so as to increase the volume of the container, the partial pressures of all of the gases in the container will . the equilibrium will shift in the direction that produces moles of gas.
Answers
The equilibrium will change to favour the direction that generates more moles of gas as the volume increases.
What happens when an equilibrium with a fixed volume has an inert gas injected to it?When the inert gas is introduced under these circumstances, the overall pressure rises but the concentration of the reactants and products stays the same. When the equilibrium condition and volume are unchanged, the addition of inert gas has no impact on the equilibrium constant.
The equilibrium will move to the side of the reaction where there are less moles of gas as pressure rises. The equilibrium will move toward the side of the reaction where there are more gas molecules when the pressure is reduced.
learn more about equilibrium condition refer
https://brainly.com/question/517289
#SPJ4
how does ease of ion pair formation depend on concentration.
Answers
Can you help me with this question
Please do all the parts a through d
And have good explanation

Answers
a) According to the balanced equation, for every 5 moles of Br that react, 3 moles of Br₂ are produced. Therefore, the rate of appearance of Br₂ will also be 3.5 × 10⁻¹ mol L⁻¹s⁻¹.
b) For every 6 moles of H⁺ that react, 3 moles of H₂O are produced. Therefore, the rate of appearance of H₂O will be half the rate of disappearance of H⁺, which is 1.75 × 10⁻¹ mol L⁻¹s⁻¹.
c) According to the balanced equation, for every 6 moles of H⁺ that react, 5 moles of Br and 1 mole of BrO₃⁻ are consumed. Therefore, the rate of disappearance of H⁺ will be (5/6) × 3.5 × 10⁻¹ mol L⁻¹s⁻¹, which is 2.92 × 10⁻¹ mol L⁻¹s⁻¹.
d) According to the balanced equation, for every 1 mole of BrO₃⁻ that reacts, 3 moles of Br₂ are produced. Therefore, the rate of disappearance of BrO₃⁻ will be (1/5) × 3.5 × 10⁻¹ mol L⁻¹s⁻¹, which is 7.0 × 10⁻² mol L⁻¹s⁻¹.
Depending on the type of reactants, their concentration, temperature, and other variables, chemical reactions proceed at varying speeds. We can comprehend and measure the rates of these reactions with the aid of kinetics.
In this issue, the rates of the various species contributing to the chemical reaction are to be determined.
By doing this, we can learn more about the variables that influence this reaction's rate and possibly improve the conditions for the creation of the desired goods more quickly or effectively.
We found that the rate of appearance of Br₂ is also 3.5 × 10⁻¹ mol L⁻¹s⁻¹, the rate of appearance of H₂O is 1.75 × 10⁻¹ mol L⁻¹s⁻¹, the rate of disappearance of H⁺ is 2.92 × 10⁻¹ mol L⁻¹s⁻¹, and the rate of disappearance of BrO₃⁻ is 7.0 × 10⁻² mol L⁻¹s⁻¹.
learn more about rate of disappearance here
https://brainly.com/question/13762874
#SPJ1
What are the similarities and differences between ionic and covalent bonds?
Answers
Answer:
Explanation:
Similarities.
Both Ionic and covalents bond produce exothermic reactions.
They are both neutral.in Ionic bonds, the two opposite charge will terminate each other and in covalent, the neutral molecules tend to share electrons.
Difference
Ionic bonds have high polarity while covalent have low.
Ionic bonds have no definite shape, covalent have.
Ionic have high melting points, covalent have low.
Io ic have high boiling point, covalents have low.
PLEASE HELP !!!!!!!!!!!!!!!!!!!!!!!!!!!!!
Which of the following does NOT increase with increasing temperature?
a kinetic energy
b thermal energy
c potential energy
d none of the above
Answers
Answer:
Explanation:
Potential energy
What is the atomic number for an element whose mass number is 78,
which contains 30 neutrons per atom?
Answers
Answer:
protons = atomic numbermass number = protons + neutronsatomic number = mass number - neutrons = 78-38 = 40
Explanation:
The atomic number of an element represents the number of protons in its nucleus.
To determine the atomic number, subtract the number of neutrons from the mass number.
In this case, the mass number is 78, and there are 30 neutrons per atom. So, atomic number = mass number - number of neutrons = 78 - 30 = 48.
Therefore, the element with a mass number of 78 and 30 neutrons per atom has an atomic number of 48. The element with atomic number 48 is cadmium (Cd), which means it has 48 protons in its nucleus.
Know more about protons:
https://brainly.com/question/29248303
#SPJ3
When magma goes down into the lower part of the mantie where it is recycled and may come right back up the ocean ridge is called..

Answers
Calculate the standard entropy change
C2H2 (g) + 2H2 (g) → C2H6 (g
C2H2= 201
H2=131
C2H6 = 230
Answers
Entropy is a notion that essentially refers to the universe's propensity for chaos or the spontaneous changes that take place in everyday happenings. Here the standard entropy change for the given reaction is -233.
Entropy is typically referred to as a measurement of a system's randomness or disorder. In the year 1850, a German physicist by the name of Rudolf Clausius first proposed this idea. Entropy is a thermodynamic property that is used to characterize how a system behaves in terms of temperature, pressure, entropy, and heat capacity.
Here the standard entropy change is:
Entropy of products - entropy of reactants
ΔS = 230 - (201 + 2 ( 131)) = -233
To know more about entropy, visit;
https://brainly.com/question/17172535
#SPJ1
A characteristic used to describe something is called a
Answers
adjectives that can be used to describe characteristics of people are called.
A metal carbonate, XCO3 of mass 2.012 g was heated resulting in the formation of XO, a metal oxide and carbon dioxide with a mass of 0.855 g according to the reaction shown below: XCO3 (s) → XO (s) + CO2 (g) (Atomic mass of O-15.999 g/mol; H-1.008 g/mol; C-12.011 g/mol).
Answers
The metal X has an approximate molar mass of 42.36 g/mol and the metal is most likely calcium.
What is the molar mass of XCO₃?The molar mass of the metal carbonate XCO₃ and identify the metal X, we need to calculate the number of moles of XCO₃ and CO₂ using the given masses and molar masses.
The molar mass of CO₂ (carbon dioxide) is 12.011 g/mol (for carbon) + 2 * 15.999 g/mol (for oxygen) = 44.01 g/mol.
The number of moles of CO₂ can be calculated using the formula:
moles of CO₂ = mass of CO₂ / molar mass of CO₂
moles of CO₂ = 0.855 g / 44.01 g/mol
moles of CO₂ ≈ 0.01944 mol
Since the reaction stoichiometry is 1:1 between XCO₃ and CO₂, the number of moles of XCO₃ is also approximately 0.01944 mol.
molar mass of XCO₃ = mass of XCO₃ / moles of XCO₃
molar mass of XCO₃ = 2.012 g / 0.01944 mol
molar mass of XCO₃ ≈ 103.38 g/mol
The molar mass of XCO₃ is approximately 103.38 g/mol.
To determine the metal X:
molar mass of X = molar mass of XCO3 - molar mass of CO3
molar mass of X = 103.38 g/mol - (12.011 g/mol + 3 * 15.999 g/mol)
molar mass of X ≈ 42.36 g/mol
Metal X is most likely Calcium that has a molar mass of 40 g/mol
Learn more about molar mass at: https://brainly.com/question/837939
#SPJ1
Which choice is not an example of a molecule? Responses H2S uppercase H subscript 2 end subscript uppercase S, O3 uppercase O subscript 3, KOH uppercase K O H, Mn uppercase M lowercase n,
Answers
From the given choice of substances, the one that is not a molecule is Mn.
What are molecules?Molecules are defined as the smallest unit of a substance that can exist and still retain the properties of that substance.
Molecules of substance are usually formed from the atom of the substances when two or more atoms of the same elements or different elements combine.
Molecules of substances are capable of existing alone but atoms of an element must combine to form molecules.
Considering the given substance to determine whether they are molecules or not:
H₂S is a molecule because it contains more than one atom of elements.
O₃ is a molecule because it contains more than one atom of elements.
KOH is a molecule because it contains more than one atom of elements.
Mn is a not molecule because it contains only one atom of elements.
Learn more about molecules at: https://brainly.com/question/475709
#SPJ1
contrast the earliest scientist's understanding of the atom with that of the most recent in your list
Answers
Our current understanding of the atom is based on Dalton's early 19th-century idea of atomism, which was developed through meteorological investigations. John Dalton is well recognised for developing the atomism hypothesis.
When British chemist John Dalton realised that compounds usually had whole number ratios of atoms, he provided the first modern proof for the existence of atoms. Because of this, it's H2O rather than H20. A novel state of matter has been demonstrated by scientists: an electron circles a nucleus at a considerable distance while being bonded to several other atoms inside the orbit. The Bose-Einstein condensate's electron (blue) orbits the nucleus (red), and the orbit encompasses a large number of other atoms (green). Chemistry's fundamental building component is an atom. It is the lowest fraction of substance into which electrically charged particles cannot be released.
learn more about atoms here:
https://brainly.com/question/13654549
#SPJ4
Using the metric "stairs" convert the following:

Answers
Trust converter
Gear converter
Trigger converter
Hope it helps
What do all of these have in common? 1 mol Fe, 1 mol Cu, 1 mol N
Answers
Answer:
1 mol
Explanation:
They all have 1 mol with Fe containing 1 mol Cu containing 1 mol and N containing 1 mole
Te equation in question 19 is an example of which type of reaction?
Answers
Answer:
algebraic reaction is the answer of this question
In the adjoining figure, the circles with the + sign represent protons and the empty circles represent neutrons. Determine which atoms in the figure are isotopes of each other.
Answers
Answer:
2 and 3 explanation in the comments
Pewter is a solidified solution of tin and lead or tin and zinc. In both cases, tin is the main component. Which metal would you classify as the solute in each type of pewter?
Answers
If you had 6H2 molecules and 4O2 molecules, how many H2O molecules could you produce?
Answers
Answer:
6
Explanation:
As , 2H2 + O2 = 2H2O
with 6 H2, 4O2 is excess.
H2O molecules formed = 6
How many moles of Ne contain 61,000,000 atoms?
Answers
Answer:
1.01×10⁻¹⁶ moles (to 3 significant figures)
Explanation:
Using avocado's constant, we can use it to find the number of moles (x) for 61,000,000 atoms of Ne:
1 mole = 6.02×10²³ atoms
x = 61,000,000 atoms
We can solve this by cross-multiplying:
6.02×10²³ × x = 1 × 61,000,000
6.02×10²³x = 61,000,000
To solve for x, divide both sides by 6.02×10²³x:
\( \frac{6.02×10²³x}{6.02×10²³} = \frac{61,000,000}{6.02×10²³ } \)
This cancels out both 6.02×10²³ on the left
\(x = \frac{61,000,000}{6.02×10²³ } \)
\(x = 1.01 \times 10⁻¹⁶\)
Calculate the molarity of the following solution: 1.0 mole of KCI in 750.0 mL of
solution.
99 M
0.750 M
B 2.0 M
1.3 M
Answers
1.3 mol ⋅ L − 1
Molarity=
Moles of solute/ Volume of solution