True or False: One example of a chemical reaction is when H20 (water) melts and changes state from ice to liquid.
Answers
Answer:
False
Explanation:
Water melting from ice into a liquid is not a chemical reaction or chemical change. It is merely a phase change from solid to liquid.
The melting of water from ice to liquid is never a chemical reaction.
Chemical reactionsChemical reactions are reactions that involve the formation of new products from the reactants.
It is as opposed to physical changes in which substances just change their states and can be returned back to the original state with time.
The melting of ice to liquid water is a physical change. The water can be returned back to ice by a process known as freezing.
More on chemical reactions can be found here: https://brainly.com/question/1689737
Related Questions
In an experiment you find the density of water to water to be 1.23 g.Ml.The theoritical value of the water's density is 1. 00 g/ml. Find the percent error
Answers
Answer:
The percent error is 23%
Explanation:
To find the percent error for the experiment carried out,
Percent error is given by the formula below
\(PE = \frac{AE}{TV}\) ×\(100\)%
Where PE is the percent error
AE is the Absolute error
TV is the Theoretical value
Also, Absolute error (AE) can be determined from
Absolute error (AE) = /Theoretical value(TV) - Experimental value (EV)/
Now, from the question,
Experimental value (EV) = 1.23 g/mL
Theoretical value (TV) = 1.00 g/mL
From,
Absolute error (AE) = /Theoretical value(TV) - Experimental value (EV)/
Absolute error (AE) = /1.00 g/mL - 1.23 g/mL/
Absolute error (AE) = / - 0.23 g/mL/
Absolute error (AE) = 0.23 g/mL
This is the Absolute error
Now, for the Percent error
\(PE = \frac{AE}{TV}\) ×\(100\)%
\(PE = \frac{0.23}{1.00}\) ×\(100\)%
\(PE = 23\) %
Hence, the percent error (PE) is 23%
How does the temperature change when a layer of glass is added?
Answers
Answer:
thermal shock
Explanation:
the temperatures inside the glass jar should have continued to increase over time. Internal stresses due to uneven heating. This is also known as “thermal shock”.
In general, the thicker the glass, the more prone it will be to breaking due to the immediate differences in temperature across the thickness of glass.
Borosilicate glass is more tolerant of this, as it has a higher elasticity than standard silicon glass.
You may also note that laboratory test tubes and flasks are made with thinner walls, and of borosilicate glass, when designated for heating.
how many xe atoms are there in a 94.6 gram sample of xe ?
Answers
In a 94.6 gram sample of Xe there are 4.335 × 10^23 Xe atoms.
Avogadro's numberTo find the number of Xe atoms in a 94.6 gram sample of Xe, we can use the formula:
number of atoms = (mass of sample) / (molar mass of Xe) × (Avogadro's number)
The molar mass of Xe is 131.29 g/mol, and Avogadro's number is 6.022 × 10²³ atoms/mol.
Plugging in the given values, we get:
number of atoms = (94.6 g) / (131.29 g/mol) × (6.022 × 10^23 atoms/mol)
Simplifying, we get:
number of atoms = (0.72 mol) × (6.022 × 10^23 atoms/mol)
number of atoms = 4.335 × 10²³ atoms
Therefore, there are 4.335 × 10²³ Xe atoms in a 94.6 gram sample of Xe.
Learn more about Avogadro's number https://brainly.com/question/14138110
#SPJ11
Fill in the [?]:
1,516,000,000 nm = [?]m
Give your answer in standard form.
Answers
Answer:
1nm = 1000000 mm 1cm=10mm 1m=100cm
Explanation:
so
1,516,000,000 nm = divided by 1000000 is 1516 mm divided by 10 = 151.6 cm divided by 100 = 0.1516 m
0.1516 = 1.516 x 10 -1
because it is 10 less than 1.516
5) To check the accuracy of our results we will compare our results to the label on the vinegar bottle. The bottle contains 4% vinegar. We will need to change our M results to %% in order to calculate a percent error.
Using the average M and the average volume (you have to change it to LITERS) of the acetic acid find the # of moles of acetic acid using the molarity formula from Table T.
Change moles to grams using the gfm of acetic acid (HC,H,O,).
Divide grams of acetic acid by the average volume (this time in ml.) of acetic acid and then multiply by 100. This is your experimental %.
Calculate the % error.
6. What other indicator could we have used?
7. What adjustment to our calculations would we have needed to make if we used barium hydroxide rather than sodium hydroxide? (It might be helpful to write the formula for barium hydroxide
Answers
5) Convert molarity to percent, calculate moles of acetic acid, convert moles to grams, divide grams by volume in mL, multiply by 100 to obtain experimental percent, and calculate percent error.
6) Phenolphthalein could have been used as an alternative indicator.
7) When using barium hydroxide instead of sodium hydroxide, adjust the calculations by considering the stoichiometry of the reaction and using a molar ratio of 2:1 between acetic acid and barium hydroxide.
5. To calculate the percent error in the concentration of acetic acid, we need to convert our molarity (M) results to percent (%). Using the average molarity and the average volume (converted to liters) of acetic acid, we can calculate the number of moles of acetic acid.
Then, by converting moles to grams using the molar mass of acetic acid (CH3COOH), we can divide the grams of acetic acid by the average volume (in milliliters) of acetic acid and multiply by 100 to obtain the experimental percent.
Finally, we can calculate the percent error by comparing the experimental percent to the labeled percent (4% vinegar on the bottle).
6. An alternative indicator that could have been used is phenolphthalein. Phenolphthalein is commonly used in acid-base titrations and changes color in a specific pH range, indicating the endpoint of the reaction.
6. If barium hydroxide (Ba(OH)2) were used instead of sodium hydroxide (NaOH), the adjustment in calculations would involve the stoichiometry of the reaction. The balanced chemical equation for the reaction between acetic acid and barium hydroxide is:
2CH3COOH + Ba(OH)2 → Ba(CH3COO)2 + 2H2O
The molar ratio between acetic acid and barium hydroxide is 2:1. Therefore, the number of moles of barium hydroxide used would be half the number of moles of acetic acid in the calculation.
The rest of the procedure, including converting moles to grams and calculating the percent, would remain the same.
For more such questions on molarity visit:
https://brainly.com/question/30404105
#SPJ8
Oxygen and chlorine gas are mixed in a container with partial pressures 5 points
of 601 mmHg and 0.739 atm, respectively. What is the total pressure inside
the container (in atm)?
Answers
Answer:
o.99
Explanation:
how does the name of CaS
differ from the name of CdS?
Answers
The names of the compounds differ by the type of metal present in them, which are Calcium (Ca) and Cadmium (Cd)
From the question,
We are to determine the how the name of CaS differ from the name of CdS
First, we will determine the identities of the elements in the given compounds
For CaSCa represents calcium
and
S represents sulfur
∴ The compound is named Calcium sulfide
For CdSCd represents the element Cadmium
and
S represent the element Sulfur
∴ The compound is named Cadmium sulfide
Hence, the names of the compounds differ by the type of metal present in them, which are Calcium (Ca) and Cadmium (Cd)
Learn more here: https://brainly.com/question/19078303
Please help How many moles of a gas sample are in 5.0 L container at 215 K and 342 kPa(The gas constant is 8.31 L kPa/mol K) Round your answer to one decimal place and enter the number only with no units.

Answers
Answer
1.0 mol
Explanation
Given:
Volume, V = 5.0 L
Temperature, T = 215 K
Pressure, P = 342 kPa
The gas constant, R = 8.31 L kPa/mol K
What to find:
The number of moles of the gas sample.
Step-step-solution:
The number of moles of the gas can be determine using the ideal gas equation formula:
\(PV=nRT\)Put the given values into the formula and calculate for n:
\(\begin{gathered} 342\times5.0=n\times8.31\times215 \\ 1710=1786.65n \\ \text{Divide both sides by 1786.65} \\ \frac{1710}{1786.65}=\frac{1786.65n}{1786.65} \\ n=0.9571\text{ mol} \\ To\text{ one decimal place,} \\ n=1.0\text{ mol} \end{gathered}\)The number of moles of the gas sample is 1.0 mol.
Give an example of 2 organisms and their adaptations. Explain how that adaptation helps them survive
Answers
One example of two organisms and their adaptations are the polar bear and the arctic fox, both of which inhabit the harsh, icy environment of the Arctic.
The polar bear has a thick layer of insulating fur, which helps it retain body heat in the cold temperatures. It also has large paws that act like snowshoes, allowing it to move over snow and ice more easily.
The polar bear's sense of smell is also highly developed, allowing it to detect prey from far distances. These adaptations help the polar bear survive in its cold, snowy habitat by keeping it warm and aiding in its ability to hunt for food.
The arctic fox also has several adaptations that allow it to survive in the Arctic. Its fur changes color from brown in the summer to white in the winter, providing camouflage against the snow. The fox's paws are covered in fur to provide insulation and better traction on the slippery ice.
The fox's diet is also highly adaptable, allowing it to survive on a variety of prey, from lemmings to birds to fish. These adaptations help the arctic fox survive in the harsh Arctic environment by helping it blend in, move more efficiently, and find food.
Learn more about polar bear here:
https://brainly.com/question/21898980
#SPJ1
What is the molarity of a solution made from dissolving 3.0 moles of Saline (NaCl) in 0.25 L of solution?
Answers
Answer: the answer is 12M
Explanation: 3.0mol/ 0.25L =12M
12M is the molarity of a solution made from dissolving 3.0 moles of Saline (NaCl) in 0.25 L of solution.
What is molarity?The amount of molecules of molecules divided by the quantity of liters in solution is the definition of molarity, a concentration unit in chemistry. It is crucial to comprehend how it is calculated as well as when to employ it in comparison to other units because it is among the most widely used concentration units.
A chemical solution's concentration is measured in molarity (M). It refers to the solute's moles per liter of solution. To be clear, this is not the same as the liters of solvents (a common mistake).
molarity = number of moles of solute/ volume of solution in litre
molarity =3.0mol/ 0.25L
=12M
Therefore, 12M is the molarity of a solution made from dissolving 3.0 moles of Saline (NaCl) in 0.25 L of solution.
To know more about molarity, here:
https://brainly.com/question/29884686
#SPJ2
A chemist wants to extract copper metal from copper chloride solution. The chemist places 1. 50 grams of aluminum foil in a solution of 14 grams of copper (II) chloride. A single replacement reaction takes place. What best explains the state of the reaction mixture after the reaction?
Unbalanced equation: CuCl2 + Al → AlCl3 + Cu
Less than 6. 0 grams of copper is formed, and some aluminum is left in the reaction mixture.
More than 6. 5 grams of copper is formed, and some aluminum is left in the reaction mixture.
Less than 6. 0 grams of copper is formed, and some copper chloride is left in the reaction mixture.
More than 6. 5 grams of copper is formed, and some copper chloride is left in the reaction mixture
Answers
Less than 6. 0 grams of copper i.e. 0.36 g is formed, and some aluminum is left behind in the reaction ingredients.
The amount of copper made is calculated from the balanced equation of the reaction.
The balanced equation :
3 CuCl2 + 2 Al → 2 AlCl3 + 3 Cu
molar mass of CuCl₂ = 132 g/mol
molar mass of Al = 27.0 g
molar mass of Cu = 64.0 g
moles of aluminium = 0.50 / 27 = 0.018 moles
moles of CuCl₂ produced = 0.75/132 = 0.00568 moles
So, CuCl₂ is acting as the limiting reagent in the reaction.
3 moles of CuCl₂ makes 3 moles of Cu
Similarly,
0.00568 moles CuCl₂ will make 0.00568 moles of Cu
Mass of Cu produced = 0.00568 * 64 = 0.36 g
So, Less than 6. 0 grams of copper is formed, and some aluminum is left in the reaction mixture as CuCl2 acts as limiting reagent.
Learn more about Copper
brainly.com/question/13677872
#SPJ4
If Steve throws the football 50 meters in 5 seconds, what is the average speed of the football?
Answers
Answer:
10 meters per second
Explanation:
When air pressure is high what is the weather like
Answers
Answer:
high-pressure systems normally associate with dry weather and mostly clear skies. This usually brings some light winds of cool, dry air, and brings fair weather.
For each of the following substances below, identify the type of bonding between the atoms. Justify your
reasoning

Answers
Answer:
SF4 -Covalent bond
BaBr2 - Ionic bond
Ca - Metallic bond
Explanation:
Sulphur and fluorine are non metals. Nonmetal atoms combine by the sharing of electrons. This is also called a covalent combination(bond).
In BaBr2, a metal is combined with a nonmetal. This combination involves a transfer of electrons from the metal to the nonmetal forming an ion pair. This is otherwise called an ionic bond.
Atoms of a metal are held together by strong metallic bonds that involves positively charged cations and a cloud of electrons.
Suppose that 5.2 L of methane at a pressure
of 782 Torr is transferred to a vessel of volume
2.2 L. What is the final pressure of methane
if the change occurs at constant temperature?
Answer in units of Torr.
Answers
Answer:
Final pressure = 1848.36 Torr
Explanation:
Given that,
Initial volume, V₁ = 5.2 L
Initial pressure, P₁ = 782 Torr
Initial volume, V₂ = 2.2 L
We need to find the final pressure. We know that the relationship between pressure and volume is given by :
\(P\propto \dfrac{1}{V}\\\\\dfrac{P_1}{P_2}=\dfrac{V_2}{V_1}\\\\P_2=\dfrac{P_1V_1}{V_2}\\\\P_2=\dfrac{782\times 5.2}{2.2}\\\\P_2=1848.36\ torr\)
So, the final pressure is equal to 1848.36 Torr.
How does the radius of calcium compare to the radius of potassium?
Answers
Answer:
Calcium has a smaller radius in comparison to Potassium.
Explanation:
When we move down a group, the atomic radius increases, and when we move from left to right in a period, the atomic radius decreases. Since both Potassium and Calcium are in the 4th period, we have to look left to right. Calcium is to the right of Potassium making the radius smaller.
explain about the degeneracy of the d9 ground electron configurations using Cu2+ as an example
Answers
Degeneracy refers to the situation where different electron orbitals have the same energy level. In the case of the d9 ground electron configuration, this occurs when there are nine electrons occupying the five available d orbitals.
Using Cu2+ as an example, let's break down the steps to understand its d9 ground electron configuration:
1. Identify the atomic number of Cu (copper): The atomic number of copper is 29, which means it has 29 electrons in its neutral state.
2. Determine the electron configuration for Cu: The electron configuration for Cu in its neutral state is [Ar] 3d10 4s1.
3. Identify the Cu2+ ion: The Cu2+ ion is formed when copper loses two electrons, specifically from its 4s orbital. This results in the electron configuration [Ar] 3d9 for Cu2+.
4. Analyze the d9 ground electron configuration: In the Cu2+ ion, there are nine electrons occupying the five available d orbitals. These orbitals are degenerate, meaning they have the same energy level. The configuration can be represented as follows: (↓↑) (↓↑) (↓↑) (↓↑) (↓). Each parenthesis represents an individual d orbital, with two electrons (↓↑) filling four of the orbitals and a single electron (↓) in the fifth orbital.
In summary, the degeneracy of the d9 ground electron configuration using Cu2+ as an example refers to the fact that the nine electrons occupy the five available d orbitals with the same energy level.
To know more about Degeneracy, visit:
https://brainly.com/question/15873781
#SPJ11
Removing thermal energy from liquid water can cause it to change to what state? (Select all correct answers)
Group of answer choices
A liquid
B solid
C it doesn't change
D ice
Answers
when a metal reacts with an acid why will the mass decrease
Answers
Acid + Metal + Salt + Hydrogen. When a metal is placed in acid, it gradually shrinks as it is consumed by the chemical reaction.
Why does magnesium reacting with hydrochloric acid cause the mass to decrease?The magnesium or acid are gradually depleted throughout this reaction. Nevertheless, because there is an excess of acid, the shift in rate is mostly brought on by the loss in magnesium (because the surface area decreases).
Why does mass go up following a reaction?This is so because the atoms from the reactants were used to form the new substances. To create the products, the atoms from of the reactants dissociate, rearrange, or re-bond in a new configuration.
To know more about Hydrogen visit:
https://brainly.com/question/28937951
#SPJ1
what does the type of reaction does the formation of adipic acid from cyclohexanol involve
Answers
The formation of adipic acid from cyclohexanol involves a chemical reaction known as oxidation. Specifically, it undergoes a two-step oxidation process.
The first step involves the oxidation of cyclohexanol to form cyclohexanone. This oxidation is typically carried out using an oxidizing agent such as sodium or potassium dichromate in the presence of sulfuric acid or a catalyst such as pyridinium chlorochromate. In this reaction, the hydroxyl group (-OH) of cyclohexanol is converted into a carbonyl group (C=O) in cyclohexanone.
The second step is the further oxidation of cyclohexanone to adipic acid. This step usually involves the use of nitric acid or nitric acid in the presence of a catalyst. In this reaction, the carbonyl group of cyclohexanone is oxidized to form two carboxyl groups (-COOH) in adipic acid.
Overall, the formation of adipic acid from cyclohexanol involves an oxidation reaction where the hydroxyl group of cyclohexanol is first converted to a carbonyl group, and then the carbonyl group is further oxidized to carboxyl groups, resulting in the formation of adipic acid.
To learn more about adipic acid click here:
brainly.com/question/14121861
#SPJ11
fill in the blanks am giving brainliest and thanx
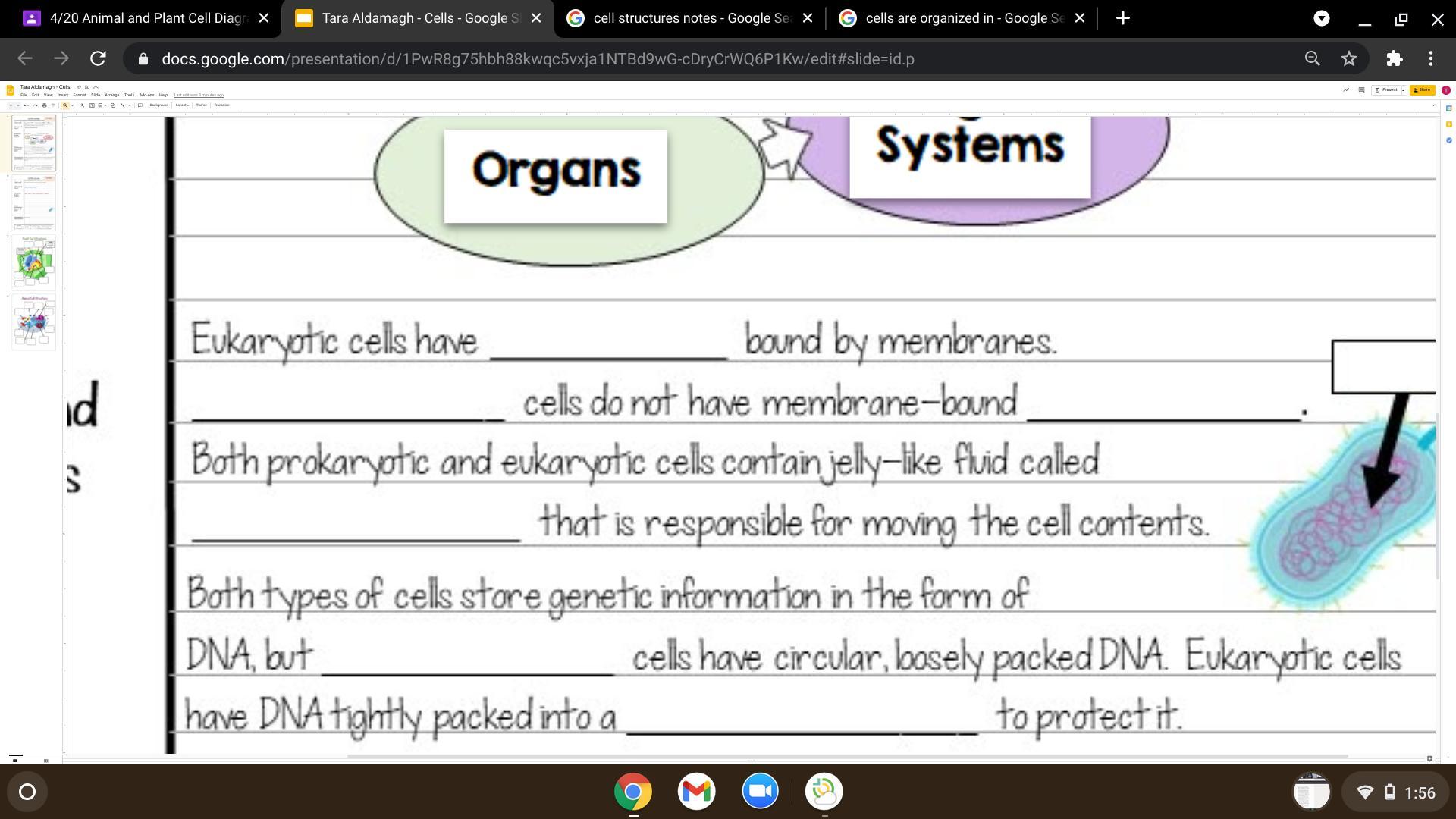
Answers
Answer:
Organelles, prokaryotic, organelles, cytoplasm, prokaryotic, nucleus
Explanation:
Organelles are membrane bound only in eukaryotic ccells.
Prokaryotic cells unlike eukaryotic cells, do not have membrane bound organlles.
Something that is found in both prokaryotic and eukaryotic cells is the cytoplasm, which is fluid that is inside the whole cell.
Unlike eukaryotic cells, prokaryotic cells have loose DNA, which floats around the cell quite literally.
The only way DNA is stored in eukaroyotic cells is in the nucelus, which is only found in eukaryotic cells, not prokaryotic.
Hope this helps ;)
Calculate the pH for each of the cases in the titration of 35.0 mL of 0.180 M KOH(aq) with 0.180 M HI(aq). Note: Enter your answers with two decimal places.
Answers
The pH at the equivalence point is 7.00, before the equivalence point is 0.74 (basic), and after the equivalence point is 0.74 (acidic).
In this titration, we have a strong base (KOH) reacting with a strong acid (HI). At the equivalence point, all the KOH will have reacted with HI to form KI and H₂O. We can use the stoichiometry of this reaction to calculate the number of moles of HI needed to reach the equivalence point.
First, we need to determine the volume of HI needed to reach the equivalence point. Since we have 35.0 mL of 0.180 M KOH, we can use the equation M1V1 = M2V2 to find the number of moles of KOH present:
0.180 M x 0.0350 L = 0.00630 mol KOH
Since the reaction between KOH and HI is 1:1, we need 0.00630 moles of HI to reach the equivalence point. Using the same equation, we can find the volume of HI needed:
0.180 M x V(HI) = 0.00630 mol HI
V(HI) = 0.0350 L
At the equivalence point, the solution will contain only KI and water. The pH of this solution will be neutral, or 7.00.
Before the equivalence point, the KOH is in excess and the solution is basic. We can use the equation for the reaction of KOH and water to calculate the concentration of hydroxide ions:
KOH(aq) + H₂O(l) → K⁺(aq) + OH⁻(aq)
The initial concentration of KOH is 0.180 M, so the concentration of OH⁻ will also be 0.180 M. Using the equation for the ion product constant of water, we can calculate the pH:
pH = -log[OH⁻] = -log(0.180) = 0.74
After the equivalence point, the HI is in excess and the solution is acidic. We can use the equation for the reaction of HI and water to calculate the concentration of hydronium ions:
HI(aq) + H₂O(l) → H₃O⁺(aq) + I⁻(aq)
The initial concentration of HI is 0.180 M, so the concentration of H₃O⁺ will also be 0.180 M. Using the equation for pH, we can calculate the pH:
pH = -log[H₃O⁺] = -log(0.180) = 0.74
Therefore, the pH at the equivalence point is 7.00, before the equivalence point is 0.74 (basic), and after the equivalence point is 0.74 (acidic).
To know more about pH, refer to the link below:
https://brainly.com/question/31132561#
#SPJ11
Which of these is an example of temporal isolation?
Answers
The example of a temporal isolation will be: "one species perform a specific courtship dance, the other species does not". Option B is correct.
Temporal isolation refers to a type of reproductive isolation in which two species reproduce at different times, which prevents them from interbreeding. This type of isolation can lead to the formation of distinct species over time, as it allows genetic differences to accumulate between populations that breed at different times.
For example, two species of frogs may live in the same area, but one species breeds in early spring while the other species breeds in late summer. Because they breed at different times, they do not interbreed, and their genetic makeup becomes increasingly different over time, eventually leading to the formation of two distinct species.
To know more about temporal isolation here
https://brainly.com/question/2410480
#SPJ4
--The given question is incomplete, the complete question is
"Which of these is an example of temporal isolation? A) One species is found only in the eastern united states, the other only in England B) one species perform a specific courtship dance, the other species does not C) One species average height is 45kg, the others average height is 290kg D) One species is a primate, the other is a marsupial E) One species is nocturnal and the other species is not."--
If a student measures the density of aluminum as 2. 87 g/mL and the actual value is 2. 69 g/mL, what is the student's % error?
Answers
If the student measures the density of the aluminum as 2. 87 g/mL and the actual value is the 2.69 g/mL, the student's % error is 6.6 %.
The measures density of the aluminum = 2.87 g/mL
The actual density of the aluminum = 2.69 g/mL
The expression of the percent error is as follows :
% error = (|actual value - measured value | / actual value ) × 100 %
% error = (|2.87 - 2.69 | / 2.69 ) × 100 %
% error = 0.066 × 100 %
% error = 6.6 %
Thus, the percent error is 6.6 %.
To learn more about percent error here
https://brainly.com/question/15983683
#SPJ4
What mass of water is required to dissolve 175 g KNO3 (potassium Nitrate) to produce a 32.25 m solution?
Answers
ANSWER
The mass of water is 0.0536 kg
STEP-BY-STEP EXPLANATION;
Given information
The mass of KNO3 = 175g
The molarity of the solution = 32.25 M
The molality formula is given below as
\(\text{ Molality = mole of solute }\div\text{ kg of solvent}\)The first step is to find the mole of the solute using the below formula
\(\text{ Mole = mass }\div\text{ molar mass}\)Recall, the molar mass of KNO3 is 101.1032 g/mol
\(\begin{gathered} \text{ Mole = 175 }\div\text{ 101.1032} \\ \text{ Mole = 1.731 moles} \end{gathered}\)The second step is to find the mass of water using the molality formula
\(\begin{gathered} \text{ Molality = mole of solute }\div\text{ kg of solvent} \\ 32.25\text{ = 1.731}\div\text{ kg of solvent} \\ \text{ cross multiply} \\ \text{ 1.731 = 32.25 }\times\text{ Kg of solvent} \\ \text{ kg of solvent = 1.731 }\div\text{ 32.25} \\ \text{ kg of solvent = 0.0536 kg} \end{gathered}\)Hence, the mass of water is 0.0536 kg
HELP PLS!!!!!!! NEED THIS IN 5 MINS
2. What kind of bond is pictured in the diagram to the left? Describe the movement of the valence electrons in the bond above? (Hint: Sharing or giving up?)
in a complete sentence pls

Answers
Answer:
Sharing or covalent bonding is taking place.
Explanation:
The valence electron will be shared by both the atoms so that means that the election will move around both of the atoms.
Please help me fast...

Answers
B) 1 and 3
it's the right answer
Answer:
For 3rd question, the answer is option D
what is the standard cell potential for a galvanic cell that consists of al3 /al and cu2 /cu half-cells?
Answers
The +2.00 V is the standard cell potential for a galvanic cell that consists of al3 /al and cu2 /cu half-cells.
What is galvanic cell ?
A galvanic cell or voltaic cell is an electrochemical device that transforms chemical energy from spontaneous redox reactions into electrical energy. Galvanic cell A voltaic cell is an electrochemical device that uses chemical reactions to produce electricity.
What is standard cell ?
A standard cell is a collection of transistor and connection structures that performs boolean logic operations (such as AND, OR, XOR, XNOR, inverters) or storage operations (flipflop or latch).
The standard half reactions are
Al3+ (aq) + 3 e- -------> Al (s); E0 (Al3+/Al) = -1.66 V
Cu2+ (aq) + 2 e- ------> Cu (s); E0 (Cu2+/Cu) = +0.34 V
Since Cu2+/Cu has a more positive standard reduction potential as compared to Al3+/Al, hence, Cu2+ will be reduced while Al will be oxidized. The half reactions comprising the redox reaction are then
Reduction: Cu2+ (aq) + 2 e- --------> Cu (s) E0red = +0.34 V
Oxidation: Al (s) -------> Al3+ (aq) + 3 e- E0ox = -E0(Al3+/Al) = -(-1.66 V) = +1.66 V
The redox reaction is given as
3 Cu2+ (aq) + 2 Al (s) -------> 3 Cu (s) + 2 Al3+ (aq)
E0cell = E0red + E0ox
= (+0.34 V) + (+1.66 V)
= +2.00 V
Therefore, +2.00 V is the standard cell potential for a galvanic cell that consists of al3 /al and cu2 /cu half-cells.
Learn more about galvanic cell from the given link.
https://brainly.com/question/13031093
#SPJ4
The picture shows two lightbulbs powered by a solar cell. Describe the
energy transfers that make the bulbs light, starting with energy from the
Sun.*
Answers
Answer:
light energy --> electric energy ----> light and heat energy by the bulb
Explanation:
The energy transfers that make the bulbs light, starting with energy from solar energy to electrical energy and from electrical energy to heat & light energy.
What is energy transfer?Transfer of energy is a process in which one form of energy will convert into other form of energy, and total energy during this conversion is conserved. When the solar energy comes from the Sun and falls on the solar panel, then PV cells that are present in the panel will absorb this solar energy and generate electrical charge. This electrical charges are responsible for the flow of internal electricity inside the cell.This generated electricity used by the bulbs to generate light energy as well as heat energy.
Hence, Solar energy will convert in to electrical energy which in turn converts into light and heat energy.
To know more about conservation of energy, visit the below link:
https://brainly.com/question/166559
describe one way the respiratory and nervous system work together
Answers
The respiratory system supplies oxygen to the blood and removes carbon dioxide. The brain monitors respiratory volume and blood gas levels. The brain regulates respiratory rate.
☆...hope this helps...☆
_♡_mashi_♡_
One way the respiratory and nervous systems work together is through the control of breathing rate and depth based on the body's oxygen and carbon dioxide levels.
Chemoreceptor Response to Gas Levels:
Specialized sensors called chemoreceptors are located in the blood vessels, particularly in the aorta and carotid arteries, which detect changes in the levels of oxygen and carbon dioxide in the bloodstream. These chemoreceptors are part of the nervous system and play a crucial role in regulating breathing.
When the oxygen level in the blood decreases or the carbon dioxide level increases (indicating a need for more oxygen or removal of excess carbon dioxide), the chemoreceptors send signals to the brainstem, specifically to the medulla oblongata and pons. These regions of the brain are responsible for controlling involuntary processes like breathing.
The nervous system, upon receiving these signals, responds by adjusting the rate and depth of breathing to ensure that the body's oxygen and carbon dioxide levels remain within a healthy range.
For example, if the oxygen level drops due to increased physical activity, the nervous system stimulates an increase in breathing rate to bring in more oxygen. Similarly, if carbon dioxide levels rise (as during intense exercise), the nervous system triggers deeper and faster breathing to expel excess carbon dioxide.
To know more about nervous systems here
https://brainly.com/question/33728156
#SPJ3