Answers
Answer:
Its true I think but I may be wrong lol
Answer:
An apple, potato, and onion all taste the same if you eat them with your nose plugged An apple, potato, and onion all taste the same if you eat them with your nose plugged An apple, potato, and onion all taste the same if you eat them with your nose plugged An apple, potato, and onion all taste the same if you eat them with your nose plugged An apple, potato, and onion all taste the same if you eat them with your nose plugged An apple, potato, and onion all taste the same if you eat them with your nose plugged An apple, potato, and onion all taste the same if you eat them with your nose plugged An apple, potato, and onion all taste the same if you eat them with your nose plugged An apple, potato, and onion all taste the same if you eat them with your nose plugged An apple, potato, and onion all taste the same if you eat them with your nose plugged An apple, potato, and onion all taste the same if you eat them with your nose plugged An apple, potato, and onion all taste the same if you eat them with your nose plugged An apple, potato, and onion all taste the same if you eat them with your nose plugged An apple, potato, and onion all taste the same if you eat them with your nose plugged An apple, potato, and onion all taste the same if you eat them with your nose plugged An apple, potato, and onion all taste the same if you eat them with your nose plugged An apple, potato, and onion all taste the same if you eat them with your nose plugged An apple, potato, and onion all taste the same if you eat them with your nose plugged An apple, potato, and onion all taste the same if you eat them with your nose plugged An apple, potato, and onion all taste the same if you eat them with your nose plugged An apple, potato, and onion all taste the same if you eat them with your nose plugged An apple, potato, and onion all taste the same if you eat them with your nose plugged An apple, potato, and onion all taste the same if you eat them with your nose plugged An apple, potato, and onion all taste the same if you eat them with your nose plugged
Explanation:
y e s
Related Questions
Safety considerations
• Sodium Hydroxide (please insert one statement of caution from MSDS)
Answers
Answer:
d took the test
Explanation:
Why do we monitor chinstrap penguins instead of krill?
Answers
Answer:Yes
Explanation:
Because Chinstrap penguins eat krills
A student borrows $10,000 from a bank but ends up paying $13,000 on the
loan over the next five years. How much interest did the student pay?
Answers
The student paid an interest of $3,000 over the next five years.
HOW TO CALCULATE INTEREST:
The interest on a borrowed amount of money can be calculated by subtracting the principal from the amount paid over time. That is;
Interest = principal - amount
According to this question, a student borrows $10,000 from a bank but ends up paying $13,000. This means that the principal is $10,000 while the amount is $13,000. The interest is calculated thus;
Interest = $13,000 - $10,000
Interest = $3,000
Therefore, the student paid an interest of $3,000 over the next five years.
Learn more about interest at: https://brainly.com/question/4626564
To compress nitrogen at 2.5 atm from 850 mL to 540 mL, what must the new pressure be of the temperature is kept constant?1)name the law first2)identify the given , write down the equation and show work
Answers
Answer:
The new pressure is 3.94atm.
Explanation:
1) The law of gases when the temperature is constant is called Boyle's law.
2)
• Identify the given
The given information is:
P1: 2.5 atm (initial pressure)
V1: 850mL (initial volume)
V2: 540 mL (final volume)
P2: this is what we have to calculate (final pressure).
• Equation, of Boyle's law
\(P_1*V_1=P_2*V_2\)• Resolution
We have to replace the given values to calculate the final pressure (P2):
\(\begin{gathered} P_1*V_1=P_2*V_2 \\ 2.5atm*850mL=P_2*540mL \\ 2,125atm*mL=P_2*540mL \\ \frac{2,125atm*mL}{540mL}=P_2 \\ 3.94atm=P_2 \end{gathered}\)So, the new pressure is 3.94atm.
how do you Calculate the molarity of 250 ml of solution in which 2.7 g of MgCl2 are dissolved
Answers
Answer:
Molar mass of MgCl2 is 95 g/mol
Mg = 24 g/mol and Cl = 35.5 ×2 = 71 g/mol
moles = mass given/ molar mass
= 2.7/95 = 0.028 mol
volume = 250/1000 = 0.25 dm3 (ml is the same as dm3)
molarity of MgCl2 = moles/volume
= 0.028/0.25
= 0.112 mol/dm3
Match the definition with the correct vocab word. - Numbers to Names
Atom
Nucleus
Electron
Neutron
Proton
Matter
1.
Anything that has mass and takes up space.
2.
The basic unit of an element. All matter is made up of atoms.
3.
A positively charged subatomic particle located at the center of an atom.
4.
A subatomic particle with no charge located at the center of an atom.
5.
A subatomic particle of an atom that is negatively charged and orbits the nucleus extremely fast.
6.
Protons and neutrons clump together at the center of an atom to form the nucleus of an atom.
Answers
Answer:
1. Matter
2. Atom
3. Proton
4. Nucleus
5. Electron
6.Neutron
What was earth’s surface like? Landmasses? First land plants
Answers
Answer:
During the early Paleozoic Era, the Earth's surface was very different from what it is today. The continents were arranged differently, forming one large supercontinent called Pangea. This landmass was surrounded by a single large ocean called Panthalassa. The climate was much warmer and wetter than it is today, with no ice caps at the poles.
The first land plants, known as bryophytes, appeared during the early Silurian Period, around 430 million years ago. These plants were small and simple, lacking roots and vascular tissue. They grew in damp environments, such as along the edges of lakes and streams. They were important in the development of soils and in the colonization of land by other organisms, such as insects and other arthropods.
0.2g of sand in two-third in liter of ethanol . What is the concentration in g per dm cube
Answers
The mass concentration of sand in the ethanol solution is 0.299 g/dm³.
What is the concentration in grams per dm³?To find the concentration in grams per cubic decimeter (g/dm³), we first need to convert the volume from liters to cubic decimeters (dm³). Since 1 liter is equal to 1 cubic decimeter, we can directly convert the volume.
Given:
Mass of sand = 0.2 g
Volume of ethanol = two-thirds liter
Converting volume to dm³:
1 liter = 1 cubic decimeter
two-thirds liter = (2/3) cubic decimeter = 0.67 dm³ (rounded to two decimal places)
Now we can calculate the concentration in g/dm³ by dividing the mass of sand by the volume in dm³:
Concentration = Mass / Volume
Concentration = 0.2 g / 0.67 dm³
Concentration ≈ 0.299 g/dm³ (rounded to three decimal places)
Learn more about mass concentration at: https://brainly.com/question/23437000
#SPJ1
which of these is not a process of chemical weathering?
1.cracked sidewalk.
2.wearing away of the inscriptions on a New York City Monument.
3.Rusty Nail.
4.dissolving of limestone which makes caves.
Answers
the empirical mass is a whole number multiple of the molar mass true or false?
Answers
Answer:
True
Explanation:
write a balanced chemical equation (smallest integer coefficients possible) for the reaction between an acid and a base that leads to the production of mg(ch3coo)2(aq). be sure to specify states such as (aq) or (s). give the names of the acid, the base, and the salt.
Answers
The balanced chemical equation for the reaction between an acid, acetic acid and base, magnesium hydroxide leads to the production of Mg(CH₃COO)₂(aq) is :
2CH₃COOH(aq) + Mg(OH)₂(s) ----> Mg(CH₃COO)₂(aq) + 2H₂O(l)
The reaction with the acid and base is below :
CH₃COOH(aq) + Mg(OH)₂(s) ----> Mg(CH₃COO)₂(aq) + H₂O(l)
acetic acid magnesium magnesium water
hydroxide acetate
reactant product
C 2 4
H 6 8
O 4 5
Mg 1 1
the reaction is not balanced the equation multiply by 2 in CH₃COOH and 2 in H₂O , we get
2CH₃COOH(aq) + Mg(OH)₂(s) ----> Mg(CH₃COO)₂(aq) + 2H₂O(l)
This is the balanced chemical equation .
To learn more about balanced chemical equation here
https://brainly.com/question/13565642
#SPJ4
WILL MARK BRAINLIST
If a car travels 60 miles for two hours, what is the average speed?
15 mi/hr
30 mi/hr
45 mi/hr
60 mi/hr
Answers
Answer:
The average speed must be 30mph so this means the car traveled for 2hrs 60/30
calculate the molar internal energy of carbon dioxide at 298.15k , taking it's translational and rotational degrees of freedom into consideration
Answers
Answer:
Explanation:
To calculate the molar internal energy of a gas at a given temperature, you need to know the molar specific heat capacities at constant volume and constant pressure for the gas. These values are typically provided in tables of thermodynamic data, which can be found in various sources such as textbooks or online. Since you mentioned that you want to take the translational and rotational degrees of freedom into consideration, you will need to use the molar specific heat capacity at constant volume, which accounts for these degrees of freedom.
Once you have the molar specific heat capacity at constant volume for the gas, you can use the equation U = Cv * T, where U is the molar internal energy, Cv is the molar specific heat capacity at constant volume, and T is the temperature in kelvins. In your case, the temperature is 298.15 K, so plugging in the appropriate values and solving for U will give you the molar internal energy of carbon dioxide at that temperature.
It's important to note that the molar specific heat capacity at constant volume is typically a function of temperature, so you will need to use the appropriate value for the temperature you are interested in. Additionally, different sources may provide slightly different values for the molar specific heat capacity, so it's always a good idea to consult multiple sources to get a sense of the range of possible values.
please help me out

Answers
Answer:
10. B
11. C
12. B
Explanation:
10. Anything above 7 is basic, 7 is neutral, and anything below 7 is acidic.
11. Sulfur, Carbon, and Hydrogen are organic.
12. monomers build macromolecules which link and form polymers.
Which if the following matters occupies more space, assuming similar number of molecules?
Answers
Assuming a similar number of molecules, the matter that occupies the most space is gas. Option C is correct.
This is because gases have no definite shape or volume, and their molecules are spread out, moving freely in all directions. As a result, gases tend to occupy the entire volume of their container and expand to fill the available space. This is known as the "kinetic molecular theory" of gases.
In contrast, solids and liquids have a definite volume and shape. Solids have a fixed shape and their molecules are packed closely together, while liquids have a variable shape and their molecules are less closely packed. As a result, both solids and liquids occupy less space than gases.
It is worth noting that the volume of a solid or liquid can change under certain conditions, such as changes in temperature or pressure. However, even under these conditions, the space occupied by a solid or liquid is still less than that occupied by a gas. Option C is correct.
The complete question is
Which of the following matters occupies more space, assuming similar number of molecules?
A. Solid
B. Liquid
C. Gas
D. Solid and gas
To know more about the Matter, here
https://brainly.com/question/30028447
#SPJ1
The irreversible isomerization A
B was carried out in a batch reactor and the following concentration time data were obtained:
Time vs Concentration data in a Batch reactor
t 0 3 5 8 10 12 15 7.5
mol/h 4 2.89 2.25 1.45 1.0 0.65 0.25 0.07
Determine the reaction order,
, and the specific reaction a rate constant, k, using any method of your choice.

Answers
The reaction order and specific reaction rate constant can be determined by performing the kinetics experiment on irreversible polymerization A. Kinetic experiments can be used to investigate the rate and mechanism of chemical reactions. Chemical kinetics is the study of chemical reactions' speed and pathway.
The term "kinetics" refers to the study of reaction rates, which are determined by measuring the concentration of reactants and products as a function of time.Kinetics experiments can be used to determine the reaction rate and order of reaction. A chemical reaction's rate is defined as the change in the concentration of a reactant or product per unit time. The order of a reaction refers to the number of molecules that must react to produce a product. The order of reaction can be determined by measuring the initial rate of the reaction as a function of concentration.Methods for determining the reaction rate order include the initial rate method, the half-life method, and the integrated rate method. The initial rate method determines the reaction order by measuring the initial rate of the reaction at different reactant concentrations. The half-life method determines the reaction order by measuring the time it takes for the reactant concentration to decrease by half.The integrated rate method determines the reaction order by measuring the concentration of the reactant or product at different times.The specific rate constant can be determined by using the Arrhenius equation, which relates the rate constant to the activation energy, temperature, and frequency factor. The frequency factor can be determined by measuring the rate constant at different temperatures.For such more question on polymerization
https://brainly.com/question/1602388
#SPJ8
Liquid nitrogen is kept at a temperature of -320 degrees. When liquid nitrogen is heated it quickly boils and turns into a gas. Which pair of pictures represent the change caused by adding heat to liquid nitrogen?
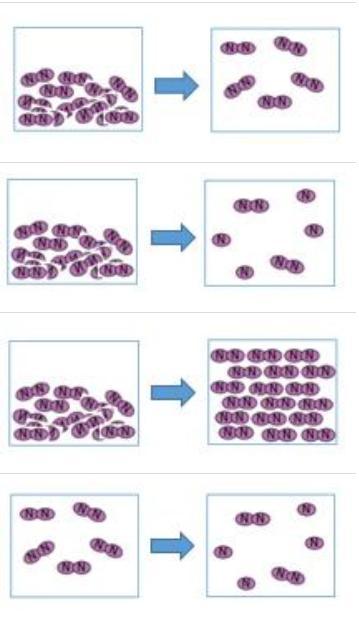
Answers
So when something is boiling it is moving faster meaning the molecules are more spread out and not condense like a solid
So the answer is the 2nd set
list five careers in science
Answers
• Biochemist
• Anthropologist
• Biologist
• Chemist
Answer:
- Psychologist
- Anthropologist
- Biochemist
- Archaeologist
- Industrial Psychologist
-Epidemiologist Scientist
-Laboratory technician
-Psychologist: A person who specializes in the study of mind and behavior or in the treatment of mental, emotional, and behavioral disorders.
-Anthropologist: A person engaged in the practice of anthropology. Anthropology is the study of aspects of humans within past and present societies. Social anthropology, cultural anthropology and philosophical anthropology study the norms and values of societies.
-Biochemist: An expert in or student of the branch of science concerning the chemical processes occurring within living organisms.
-Archaeologist: A person who studies human history and prehistory through the excavation of sites and the analysis of artifacts and other physical remains.
-Industrial Psychologist: A person who studies human history and prehistory through the excavation of sites and the analysis of artifacts and other physical remains.
-Epidemiologist Scientist: They search for the cause of disease, identify people who are at risk, determine how to control or stop the spread or prevent it from happening again.
-Laboratory technician: A medical professional who conducts lab tests on specimens and records and reports the results.
Phosphine contains 91.1 % P an 8.9% H. Water contain 88.8% of O and 11.2% of H.Phosphorus tetraoxide contains 56.4% of and 43.6% of O.Show that these data illustrate law of reciprocal proportions.
Answers
According to the law of reciprocal proportions, they should combine in the ratio of 10.24:7.92:1.29 by mass.
What is the law of reciprocal proportions ?According to the law of reciprocal proportions when two elements join to form more than one compound, the weights of one element that combine with a fixed weight of the another are in a ratio of small whole numbers.
In phosphine, phosphorus = 91.1 parts and hydrogen = 8.9 parts
So, 8.9 pads of hydrogen combine with phosphorus = 91.1 parts .
1 part of hydrogen combine = 91.1 / 8.9
= 10.24 parts
Similarly,
for water 88.8 / 11.2
= 7.92 parts of hydrogen
For Phosphorus tetra oxide
= 56.4 / 43.6
= 1.29 parts
Thus, According to the law of reciprocal proportions, they should combine in the ratio of 10.24:7.92:1.29 by mass.
To learn more about the law of reciprocal proportions, follow the link;
https://brainly.com/question/17062521
#SPJ1
Diamond and graphite are two crystalline forms of carbon. At 1 atm and 25°C, diamond changes to graphite so slowly that the enthalpy change of the process must be obtained indirectly. Determine ΔHrxn for
C(diamond) → C(graphite)
with equations from the following list:
(1) C(diamond) + O2(g) → CO2(g) ΔH = −395.4 kJ
(2) 2 CO2(g) → 2 CO(g) + O2(g) ΔH = 566.0 kJ
(3) C(graphite) + O2(g) → CO2(g) ΔH = −393.5 kJ
(4) 2 CO(g) → C(graphite) + CO2(g) ΔH = −172.5 kJ
Answers
The enthalpy change of the reaction C(diamond) → C(graphite) is -2.9 kJ.
The given information is ΔHrxn for the reaction C(diamond) → C(graphite) can be calculated with the given equations:Equations: C(diamond) + O2(g) → CO2(g) ΔH = −395.4 kJ 2 CO2(g) → 2 CO(g) + O2(g) ΔH = 566.0 kJ C(graphite) + O2(g) → CO2(g) ΔH = −393.5 kJ 2 CO(g) → C(graphite) + CO2(g) ΔH = −172.5 kJThe required reaction can be obtained by adding the equations (1) and (4), as follows:C(diamond) + O2(g) + 2CO(g) → C(graphite) + 3CO2(g)Addition of the two equations (1) and (4) results in a reaction whose products are C(graphite) and CO2.
To get the final equation that involves only the required reactants and products, the equation (2) should be added, which consumes CO2 and produces O2, as shown below:C(diamond) + O2(g) → CO2(g) ΔH = −395.4 kJ [eq. (1)] 2 CO(g) → C(graphite) + CO2(g) ΔH = −172.5 kJ [eq. (4)] 2 CO2(g) → 2 CO(g) + O2(g) ΔH = 566.0 kJ [eq. (2)] C(diamond) + O2(g) + 2CO(g) → C(graphite) + 3CO2(g) ΔHrxn=ΣΔHf(products)−ΣΔHf(reactants) ΔHrxn=[(3 mol CO2)(-393.5 kJ/mol) + (1 mol C(graphite))(0 kJ/mol)] − [(1 mol C(diamond))(0 kJ/mol) + (1 mol O2)(0 kJ/mol) + (2 mol CO(g))(−172.5 kJ/mol)] − [(2 mol CO2)(566.0 kJ/mol)] ΔHrxn=−2.9 kJ.
for such more questions on reaction
https://brainly.com/question/11231920
#SPJ8
Here is a second order reaction A→ P. If the initial concentration of A 0.0818 M goes down 30.0% in 3.15 minutes, what is the rate constant for the reaction?
Answers
The rate constant of the second-order reaction is 0.111 M^-1 min^-1.
The given data represents a second-order reaction where the rate of the reaction is proportional to the square of the concentration of A.
The integrated form of the second-order reaction is:
1/[A]t = kt + 1/[A]0
where [A]t and [A]0 are the concentrations of reactant A at time t and time zero, respectively, k is the rate constant.
We can use the given information to calculate the rate constant (k) of the reaction for the given half-life (t1/2) of 3.15 minutes:
t1/2 = (1 / k[A]0)
Using the percentage decrease in concentration and the given initial concentration, we can calculate the concentration of A at time t:
[A]t = [A]0 - 0.30[A]0 = 0.57126 M
Substituting the given values, we get:
3.15 min = (1 / k)(0.0818 M) / (0.0818 M - 0.57126 M)
Simplifying the equation above, we can solve for k:
k = 0.111 M^-1 min^-1
Therefore, the rate constant of the second-order reaction is 0.111 M^-1 min^-1.
For such more questions on constant
https://brainly.com/question/3159758
#SPJ11
How many moles are in 75 grams of potassium
Answers
Answer:
what?
Explanation: huh?
Answer:
2
Explanation:
ughhhhhhh this shoud be long
Can someone please help me with this question. I got half of the question and I am stuck on the rest.

Answers
The mean of the data set is approximately 4.0626, and the 90% confidence interval is [4.060925, 4.064275].
What is the mean and 90% confidence interval of the given data?The sample mean (x) is calculated as follows:
x = (4.0620 + 4.0550 + 4.0650 + 4.0740 + 4.0550 + 4.0660) / 6
x ≈ 4.0626 (rounded to four decimal places)
The 90% confidence interval is calculated as follows;
Standard deviation (s):
(4.0620 - 4.0626)² = 0.00000036
(4.0550 - 4.0626)² = 0.00000576
(4.0650 - 4.0626)² = 0.00000006
(4.0740 - 4.0626)² = 0.00001328
(4.0550 - 4.0626)² = 0.00000576
(4.0660 - 4.0626)² = 0.00000012
average of the squared differences:
(0.00000036 + 0.00000576 + 0.00000006 + 0.00001328 + 0.00000576 + 0.00000012) / 6 ≈ 0.00000624
s = √(0.00000624)
s ≈ 0.002496
the standard error of the mean (SEM):
SEM = 0.002496 / √6
SEM ≈ 0.001018
For a 90% confidence interval, the z value is approximately 1.645.
ME = 1.645 * 0.001018 ≈ 0.001675
CI = x ± ME
CI = 4.0626 ± 0.001675
CI ≈ [4.060925, 4.064275]
Learn more about mean and confidence intervals at: https://brainly.com/question/20309162
#SPJ1
help me solve it pls

Answers
The term molefraction is an important method which is used to calculate the concentration of a solution. It is mainly employed to calculate the concentration of a binary solution. Here the molefraction is 1 / 5. The correct option are D, D and B.
Molefraction of any component of a solution is defined as the ratio of the number of moles of that component to the total number of moles of the solution. The sum of molefraction of solute and solvent is one.
Here the molefraction of nitrogen = Moles of nitrogen / Total number of moles
1. 'x' of 'N' = 2 / 5 + 3 + 2 = 0.2 or 1 / 5
2. Molefraction of Argon = 0.60 / 0.40 + 0.04 + 0.60 = 0.57
Partial pressure = Molefraction × Total pressure
0.57 × 6.3 = 3.59 atm
3. 20 cm³ mixture contains:
20 × 1 mole / 22400 m³ = 8.9286 × 10⁻⁴
1 mole occupies 22400 cm³
8.9286 × 10⁻⁴ × 22400 cm³ / 1 mole = 20 cm³
Thus the correct option are D, D and B.
To know more about molefraction, visit;
https://brainly.com/question/13680920
#SPJ1
What is the change in boiling point atb in Celsius of a 0.852m solution of C6H14 in benzene?
Kb(benzene)= 2.65 degrees Celsius/m
Answers
The change in boiling point of C6H14 in benzene is 2.257 degree Celsius.
What is boiling point?
The boiling point is the temperature at which vapor pressure of the liquid is equal to the atmospheric pressure or the temperature at which the liquid phase turns into vapor state. The boiling point of pure water at sea level is 100 degree Celsius.
The change in boiling point can be calculated as,
ΔTb = Kb x m
where ΔTb is change in boiling point, Kb is Ebullioscopic constant and m is molality of the solute.
ΔTb = (2.65 degree Celsius / m) x 0.852m = 2.257 degree Celsius.
Therefore, the change in the boiling point of 0.852m solution of C6H14 in benzene is 2.257 degree Celsius.
To know more about boiling point click on the given link https://brainly.com/question/24675373
#SPJ1
Answer:
The correct answer for ACELLUS is 2.25
Explanation:
What is the triangle G at 294K for the following process at 1.0 atm?

Answers
The ΔG is 3.66 kJ.
To calculate the ΔG of a chemical reaction, it is necessary to use the ΔG formula and replace the values. Remember to convert the J into kJ before the calculation:
\(\begin{gathered} \Delta G=\Delta H-T\cdot\Delta S \\ \Delta G=31.0\frac{kJ}{mol}-294K\cdot0.093\frac{kJ}{\text{mol}\cdot K} \\ \Delta G=3.66\frac{kJ}{mol} \end{gathered}\)So, the ΔG of the reaction is 3.66 kJ.
A copper coin (0.377 J/goC) and a silver coin (0.239 J/goC) are placed out in the sun. Which will heat up faster and why?A. Silver; requires more energyC. Copper; requires more energy B. Same rate; requires same amount of energies
Answers
The change in temperature of the coins will depend on the energy they need to do so, this energy depends on the specific heat of the material. The specific heat corresponds to the energy required to raise the temperature of 1 gram of material 1 degree Celsius.
We see that copper has a higher specific heat, therefore, copper will require more energy to raise its temperature and will require more time.
So, the answer will be: C. Copper; requires more energy
Calculate the cell potential for the galvanic cell in which the given reaction occurs at 25 °C, given that [Sn2+]=0.0624 M, [Fe3+]=0.0437 M, [Sn4+]=0.00655 M, and [Fe2+]=0.01139 M. Standard reduction potentials can be found in this table.
Sn2+(aq)+2Fe3+(aq)↽−−⇀ Sn4+(aq)+2Fe2+(aq)
So far my incorrect answers have been:
0.28
0.798
0.178
0.142
0.881
0.61
and 0.812
Answers
Answer:
The cell potential for the given galvanic cell is 0.188 V.
Explanation:
To calculate the cell potential, we can use the Nernst equation:
Ecell = E°cell - (RT/nF)ln(Q)
where E°cell is the standard cell potential, R is the gas constant (8.314 J/mol·K), T is the temperature in Kelvin (25°C = 298 K), n is the number of moles of electrons transferred (in this case, n = 2), F is the Faraday constant (96,485 C/mol), and Q is the reaction quotient.
First, we need to write the half-reactions and their standard reduction potentials:
Sn4+(aq) + 2e- → Sn2+(aq) E°red = 0.15 V
Fe3+(aq) + e- → Fe2+(aq) E°red = 0.77 V
The overall reaction is the sum of the half-reactions:
Sn2+(aq) + 2Fe3+(aq) → Sn4+(aq) + 2Fe2+(aq)
The reaction quotient Q can be expressed as:
Q = [Sn4+][Fe2+]^2 / [Sn2+][Fe3+]^2
Substituting the given concentrations, we get:
Q = (0.00655)(0.01139)^2 / (0.0624)(0.0437)^2 = 0.209
Now we can calculate the cell potential:
Ecell = 0.15 V + 0.0592 V log([Fe2+]^2/[Fe3+]) + 0.0592 V log([Sn4+]/[Sn2+])
= 0.15 V + 0.0592 V log(0.01139^2/0.0437^2) + 0.0592 V log(0.00655/0.0624)
= 0.188 V
Therefore, the cell potential for the given galvanic cell is 0.188 V.
The cell potential for the given galvanic cell in which the given reaction occurs at 25 °C is 0.188 V.
How to the cell potential of galvanic cell?To find the cell potential, we take the Nernst equation:
Ecell = E°cell - (RT/nF)ln(Q)
In which R is the gas constant (8.314 J/mol·K) and E° cell is the standard cell potential.
T temperature in Kelvin (25°C = 298 K), and n is the number of moles of electrons transferred (n = 2), Q is the reaction quotient and F is the Faraday constant (96,485 C/mol).
Firstly, write the half-reactions and then their standard reduction potentials:
Sn⁴⁺(aq) + 2e⁻ → Sn²⁺(aq) E°red = 0.15 V
Fe³⁺(aq) + e⁻ → Fe²⁺(aq) E°red = 0.77 V
The overall reaction is the sum of the half-reactions:
Sn²⁺(aq) + 2Fe³⁺(aq) → Sn⁴⁺(aq) + 2Fe²⁺(aq)
The Q reaction quotient can be written as:
Q = [Sn⁴⁺][Fe²⁺]² ÷ [Sn²⁺][Fe²⁺]²
Substituting the given concentrations, we observe:
Q = (0.00655)(0.01139)² ÷ (0.0624)(0.0437)² = 0.209
Next, we can find the cell potential:
Ecell = 0.15 V + 0.0592 V log([Fe²⁺]²/[Fe³⁺]) + 0.0592 V log([Sn⁴⁺]/[Sn²⁺])
= 0.15 V + 0.0592 V log(0.01139²÷0.0437²) + 0.0592 V log(0.00655÷0.0624)
= 0.188 V
Thus, the cell potential for the given galvanic cell is 0.188 V.
Learn more about cell potential, here:
https://brainly.com/question/29719917
#SPJ2
Observe the movement of the skater during his run on the ramp click bar graph at what position is the potential energy of the skater the highest
Answers
Potential energy is stored energy that is affected by the relative location of different components of a system. When a spring is squeezed or expanded, its potential energy increases.
What is potential energy simple answer?Potential energy is the energy retained by an object as a result of its location relative to other objects, internal stresses, electric charge, or other variables. Although it has ties to the ancient Greek scholar Aristotle's idea of potentiality, the word potential energy was coined by the 19th-century Scottish engineer and physicist William Rankine.
The gravitational potential energy of an object, the elastic potential energy of a stretched spring, and the electric potential energy of an electric charge in an electric field are all examples of common kinds of potential energy. The joule, denoted by the sign J, is the measure of energy in the International System of Units (SI).
Learn more about potential energy
#SPJ1
What is the mass in grams of 5.50 moles of Copper, Cu?
Answers
Answer:
349.503 g
https://www.convertunits.com/from/moles+Copper/to/grams
here is a link, you can convert moles of copper to grams here
The answer is 5.50 moles of Cu (Copper) has 349.503 grams mass .
What is a mole ?
A mole is defined as 6.02214076 × 10²³ atoms, molecules, ions, or other chemical units.
and the molar mass of a substance is defined as the mass of 1 mole of that substance, expressed in grams per mole.
It is equal to the mass of 6.022 × 10 23 atoms, molecules, or formula units of that substance.
1 mole of Cu has 63.546 grams of Cu
So 5.50 moles will have 5.50 * 63.546 grams
=349.503 grams
Therefore 5.50 moles of Cu (Copper) has 349.503 grams mass .
To know more about moles
https://brainly.in/question/148570
#SPJ2