Titanium (Ti, #22) atoms bond with Oxygen (O, #8) atoms. What type of bond will hold them together? (1 pt)
*
1 point
ionic
covalent
metal
intermolecular (hydrogen, dipole, etc.)
Answers
Titanium (Ti,) atoms bond with Oxygen (O,) atoms. covalent type of bond hold them together.
What is covalent bond?An electron exchange that results in the formation of electron pairs between atoms is known as a covalent bond.
When atoms share electrons, a permanent equilibrium of the attractive and repulsive forces between them is known as covalent bonding.
These electron pairs are referred to as shared pairs or bonding pairs.
Thus ,Titanium (Ti,) atoms bond with Oxygen (O,) atoms. covalent type of bond hold them together.
To learn more about Covalent bond, refer to the below link:
https://brainly.com/question/11674395
# SPJ1
Related Questions
How many moles of HCl can be neutralized by 154. 9 grams of KOH ?
Answers
2.76 moles of HCl can be neutralized by 154.9 grams of KOH.
To determine how many moles of HCl can be neutralized by 154.9 grams of KOH, you need to use the stoichiometry of the balanced equation. The balanced equation for the reaction between HCl and KOH is:HCl + KOH → KCl + H2OFrom this equation, you can see that 1 mole of HCl reacts with 1 mole of KOH. Therefore, the number of moles of KOH that can react with the given mass of KOH is:mass of KOH = 154.9 gramsmolar mass of KOH = 39.1 + 16 + 1 = 56.1 g/molnumber of moles of KOH = mass of KOH/molar mass of KOH= 154.9/56.1= 2.76 mol. Therefore, 2.76 moles of HCl can be neutralized by 154.9 grams of KOH.
learn more about moles
https://brainly.com/question/12571105
#SPJ11
When cadmium (Cd) is mixed with hydrochloric acid (HCI), a single-displacement reaction occurs.
What will be the product or products?
O CaCl2
O CaCl2 + H2
O Cl and H
O Cd + HCI
Answers
Answer:is Cl and H
Explanation:
2Cd+2Hcl=2CdCl + H2
A piece of metal was heated and then
put it into 100.0 mL of water, initially at
23.7 °C. The metal and water were
allowed to come to an equilibrium
temperature, determined to be 27.8 °C.
How much energy did the water absorb?
J
CH,O = 4.18 _

Answers
The heat absorbed is 1713.8 J
What is the heat capacity?Heat capacity is the amount of heat energy required to increase the temperature of a substance by one degree Celsius (or Kelvin). We can use it as the means that we can use to identify a given substance.
Heat capacity is important in engineering thermodynamics, materials science, and process engineering.
We know that;
H = mcdT
H = 100 g * 4.18 * (27.8 - 23.7)
H = 1713.8 J
Learn more about heat capacity:https://brainly.com/question/28302909
#SPJ1
Halides in Group 17 combine in a 1:2 ratio with the alkaline earth metals in Group 2. For example, magnesium and chlorine ions combine to form magnesium chloride, MgCl2. What other elements form compounds in a 1:2 ratio with the halides
Answers
In each case, the alkaline earth metal (Group 2 element) combines with the halide (Group 17 element) in a 1:2 ratio to form a stable compound.
How is a 1:2 ratio formed with group 17 elements and others?
Alkaline earth metals in Group 2, like magnesium (Mg), commonly form compounds in a 1:2 ratio with halides. This is because Group 2 elements have a +2 charge, while Group 17 halides have a -1 charge. The 1:2 ratio balances the charges, resulting in a neutral compound. Examples of such compounds include:
1. Calcium (Ca) and chlorine (Cl) form calcium chloride (\(CaCl_{2}\)).
2. Beryllium (Be) and iodine (I) form beryllium iodide (\(BeI_{2}\)).
3. Strontium (Sr) and bromine (Br) form strontium bromide (\(SrBr_{2}\)).
4. Barium (Ba) and fluorine (F) form barium fluoride (\(BaF_{2}\)).
In each case, the alkaline earth metal (Group 2 element) combines with the halide (Group 17 element) in a 1:2 ratio to form a stable compound.
To know more about Alkaline Earth Metals:
https://brainly.com/question/29386853
#SPJ11
You test your hypothesis by completing a(n) _________.
Answers
You test your hypothesis by completing an experiment.
How we test our hypothesis?We test our hypothesis by performing experiment or research because in an experiment, we test our hypothesis and collect data in order to draw a conclusion from it.
So we can conclude that you test your hypothesis by completing an experiment.
Learn more about hypothesis here: https://brainly.com/question/11555274
#SPJ1
What are produced when a base is mixed with water?
hydrogen ions
hydroxide ions
oxygen atoms
oxygen ions
Answers
Answer:
The answer is hydrogen ions
explain why copper 2 oxide is a base although it does not turn litmus paper to blue
Answers
Answer:
See explanation
Explanation:
Copper II oxide is a base but not an alkali. An alkali is a soluble base. Since Copper II oxide is not soluble in water then it is not an alkali.
Let us recall that the change of colour of litmus with an alkali requires the presence of water. In the absence of water, solid Copper II oxide does not turn red litmus paper blue.
The ability to turn red litmus paper blue is commonly observed with alkalis and Copper II oxide is not an alkali.
Also recall that since Copper II oxide is not soluble, hydroxide ions are absent hence Copper II oxide does not turn red litmus paper blue.
What is the concentration of H₂(g), in parts per million, in a solution that contains 0.0001 g of H2(g) dissolved in 100. g of H₂O(l)?
Answers
Answer:
1 ppm :)
Explanation: i was guessing and 1 ppm was the answer :)
Detection of nitrongen ??
Answers
Answer:
The extract is boiled with FeSO4 and acidified with concentrated H2SO4. The appearance on Prussian blue colour indicates the presence of nitrogen
Explanation:
PLS mark it brainliest
CsH16 +12028CO2 +8H₂O
What is the ratio of octene (C8H16) to
oxygen in the reaction?
Answers
The ratio of octene to oxygen is 1:12.
To determine the ratio of octene (C8H16) to oxygen (O2) in the given reaction, we need to examine the balanced chemical equation. However, the equation you provided does not seem to be balanced. The coefficients for each compound must be determined to achieve a balanced equation before we can calculate the desired ratio.
Assuming you meant the combustion reaction of octene, a balanced equation would be:
C8H16 + 12O2 → 8CO2 + 8H2O
From the balanced equation, we can see that for every 1 mole of octene (C8H16), we require 12 moles of oxygen (O2) to completely react.
This means that for every 1 mole of octene, we need 12 moles of oxygen to fully combust the octene and produce the corresponding amounts of carbon dioxide (CO2) and water (H2O) as shown in the balanced equation.
For such more questions on combustion
https://brainly.com/question/13251946
#SPJ8
please help me with my question

Answers
C2H2OH is the answer
I am not sure but I think
why do only some salts dissolve ?are there any rules which tell you which will?
Answers
Answer:
The solubility of certain salts can be explained due to the small size of the particles.
Explanation:
As we know, solvents dissolve due to intermolecular spaces between two substance particles, Hence the solvent particle size should fit in those spaces.
you have 10 kg each of a radioactive sample a with a half-life of 100 years, and another sample b with a half-life of 1000 years. which sample has the higher activity?
Answers
If you have 10 kg each of a radioactive sample a with a half-life of 100 years, and another sample b with a half-life of 1000 years, sample A has a higher decay constant and higher activity.
The activity of a radioactive sample refers to the number of decays occurring per unit time. It is measured in units of becquerels (Bq) or curies (Ci). The activity of a sample is proportional to the number of radioactive nuclei present in the sample.
The decay rate of a radioactive sample is determined by its half-life. The shorter the half-life, the higher the decay rate and the higher the activity. Therefore, sample A with a half-life of 100 years will have a higher decay rate and higher activity than sample B with a half-life of 1000 years.
To calculate the activity of a sample, we use the following formula
Activity = λN
where λ is the decay constant, and N is the number of radioactive nuclei present in the sample.
Since the two samples have the same mass, the number of radioactive nuclei will be the same. Therefore, the sample with the higher decay constant (λ) will have the higher activity.
The decay constant is related to the half-life by the following formula:
λ = ln(2) / \(t^{\frac{1}{2} }\)
where ln(2) is the natural logarithm of 2, and \(t^{\frac{1}{2} }\) is the half-life.
Using this formula, we can calculate the decay constants for samples A and B
\(\lambda_{A}\)= ln(2) / 100 years = 0.00693 per year
\(\lambda_{B}\) = ln(2) / 1000 years = 0.000693 per year
To learn more about radioactive click on,
https://brainly.com/question/19820661
#SPJ4
a metal weighing 50.0g absorbs 20.0j of heat when its temperature increases by 150.0°c . what is the specific heat of the metal
Answers
Answer:
2.677 J/g°C
Explanation:
Quantity of heat required to raise the temperature of the metal,
Q = mc∆T
where m is the mass of the metal, c is the metal's specific heat capacity, and ∆T is the change in temperature that accompanies the heat transfer process
20.0J = 50.0g × c × 150°C
c = 20.0J/(50.0g × 150°C)
c = 2.677 J/g°C
nuclear reactions take place where?
a) ring of electrons, b) the entire atom, c) nucleus, d) none of these
Answers
Arrange these places in order of how safe they are to live in (safest first): Japan, Spain, Ireland, Australia, Iceland.
Answers
According to World Population Review 2022 the safest country to live are as follows.
Iceland is the safest amongst all.IrelandJapanSpainAustralia is least safe in this particular list.These countries are listed safe by calculating global peace index.
What is global peace index?This index measures the nation's and regions peacefulness and is finally reviewed by Institute of Economic and Peace (IEP).
The role of IEP is to measure peace at the worldwide and country level degree and lets us to evaluate the social, political and financial elements that create peace. Each year the Institute for Economics and Peace produces the Global Peace Index, the world’s main degree of countrywide peacefulness, rating 163 nations in line with their ranges of peace.
To know more about global peace click on:
brainly.com/question/16689991
#SPJ9
flowback waste water is disposed of in a process called deep well injection which plumps large quantities of waste water down into porous sandstone and limestone rock formations underground. what potential problems could result from this?
Answers
The potential problems associated with a deep well injection is that it can result in polluting underground water.
If there are many wells nearby, injecting wastewater into subsurface rock strata can be problematic. Consider porous sandstone, which contains minute openings. Water under high pressure, such as wastewater from fracking, can penetrate the sandstone and travel with underground water.
An injection well is employed to inject fluid underground into porous geologic formations. These subterranean structures might be anything from a modest soil layer to thick sandstone or limestone. Water, wastewater, brine (salt water), and water that has been combined with chemicals are all examples of injected fluids.
To know more about an injection well, refer to the following link:
https://brainly.com/question/4472460
#SPJ1
A double pipe parallel flow heat exchanger is used to heat cold water with hot water. Hot water (cp=4.25 kJ/kg °C) enters the pipe with a flow rate of 1.5 kg/s at 80 °C and exits at 45°C. The heat exchanger is not well insulated and it is estimated that 3% of the heat given off by the hot fluid is lost through the heat exchanger. If the total heat transfer coefficient of the heat exchanger is 1153 W/m²°C and the surface area is 5 m2, find the heat transfer rate to the cold water and the logarithmic mean temperature difference for this heat exchanger. Continuous trading terms apply. The kinetic and potential energy changes of the fluid flows are negligible. There is no contamination. The fluid properties are constant.
Answers
The heat transfer rate to the cold water is 167.51 kW, and the logarithmic mean temperature difference for this heat exchanger is 28°C.
We know that, Q = m × Cp × ΔT
Where
m = mass flow rate
Cp = specific heat capacity
ΔT = Temperature difference
Q = (1.5 kg/s) × 4.25 kJ/kg °C × (80 - 45)°CQ = 172.69 kW
As per the problem, 3% of the heat given off by the hot fluid is lost through the heat exchanger.
Thus, heat loss is 0.03 × 172.69 kW = 5.18 kW
The heat transfer rate to the cold water is given as Q1 = Q - heat loss = 172.69 kW - 5.18 kW= 167.51 kW
To find the logarithmic mean temperature difference for this heat exchanger:
The formula for LMTD is,∆Tlm = (ΔT1 - ΔT2) / ln(ΔT1 / ΔT2)
where
ΔT1 = hot side temperature difference = Th1 - Tc2
ΔT2 = cold side temperature difference = Th2 - Tc1
Tc1 = inlet temperature of cold water = 20°C
Tc2 = outlet temperature of cold water = ?
Th1 = inlet temperature of hot water = 80°C
Th2 = outlet temperature of hot water = 45°C
∆T1 = Th1 - Tc2 = 80°C - Tc2
∆T2 = Th2 - Tc1 = 45°C - 20°C = 25°C
Thus,∆Tlm = (80°C - Tc2 - 45°C) / ln[(80°C - Tc2) / (45°C - 20°C)]
∆Tlm = (35°C - Tc2) / ln(2.67[(80 - Tc2) / 25])
Now, the heat exchanger is a double pipe parallel flow heat exchanger. Thus, both hot and cold fluids have the same value of LMTD.∆Tlm = 35°C - Tc2 / ln(2.67[(80 - Tc2) / 25]) = 35°C - (47.81/ln(2.67[42.79/25]))
∆Tlm = 27.81°C which is approximately equal to 28°C
Learn more about heat exchangers at https://brainly.com/question/17029788
#SPJ11
What is the predicted shape, bond angle, and hybridization for CH3? A) trigonal planar, 120°, sp2 B) trigonal planar, 120°, sp3 C) trigonal planar, 109.5°, sp2 D) trigonal pyramidal, 120°, sp2 E) trigonal pyramidal, 109.5°, sp2
Answers
However, assuming that CH3 is a part of a larger molecule, we can predict its shape, bond angle, and hybridization based on the bonding theory.
Since CH3 has three groups of valence electrons surrounding the central carbon atom, we can predict its shape to be trigonal planar. The bond angle between each of the three hydrogen atoms and the central carbon atom is predicted to be 120°. To determine the hybridization of the carbon atom, we can count the total number of electron groups (3 bonding groups + 0 lone pairs = 3 electron groups).
Based on this, we can predict the hybridization of the carbon atom to be sp2, where the s orbital and two of the p orbitals of the carbon atom hybridize to form three equivalent sp2 orbitals that are oriented in a trigonal planar arrangement. Therefore, the answer would be option A) trigonal planar, 120°, sp2.
To know more about hybridization visit:
https://brainly.com/question/14358519
#SPJ11
Hello, can someone please please please help me with this I need this like ASAP.....


Answers
While acquired immunity is a particular, adaptable defense mechanism that evolves over time and possesses memory, innate immunity is the body's natural, non-specific defense system that offers immediate protection.
Thus, the initial line of defense against infections is innate immunity, which is present from birth. It contains chemical barriers like antimicrobial proteins and enzymes, together with physical barriers like the skin and mucous membranes, to give instant, all-purpose protection.
As a result of repeated exposure to particular pathogens, acquired immunity, often referred to as adaptive immunity, gradually develops. It is distinguished by its memory and distinctiveness. When lymphocytes (B cells and T cells) are activated, they either create antibodies or cell-mediated reactions, depending on the situation.
Learn more about the acquired immunity here:
https://brainly.com/question/31760157
#SPJ1
Predict the nature of the indicated covalent bond. polar or non-polar

Answers
The given bond is polar covalent bond.
Polar covalent bonds are covalent bonds in which the electrons are shared unequally. Nonpolar covalent bonds are covalent bonds with an equal distribution of electrons. Chemists utilise electronegativity, a relative measurement of how strongly an atom attracts electrons as it forms a covalent connection, to assess the relative polarity of a covalent bond.
Polarity characterises io3-. If a molecule's dipole moment is greater than 0, it is considered to be polar. The three I-O bonds in this combination are polar due to the difference in electronegativity between the I and O atoms. The three I-O bond moments point toward I atom because I is more electronegative than O atom.
Learn more about covalent bond here;
https://brainly.com/question/10777799
#SPJ1
When the first American astronauts were planning to walk on the Moon, they knew that the gravity on the Moon was less than the gravity on Earth. With this information, what did the astronauts expect to be MOST different on the Moon? Select one: a. their mass b. their height c. their weight d. their volume
Answers
Answer:
Their weight.
Explanation:
Weight is the measurement of gravity pushing you down, so with less gravity there would be less weight.
The astronauts expect their weight to be different on the Moon
Definition of weightWeight is simply defined as the gravitational pull of the earth on an object. It is measured in Newton.
Description of weightThe weight of an object varies with location because of gravity.
The weight of an object is related to it's mass according to the following equation:
Weight = mass × Acceleration due to gravity
Since the gravity of the Moon is lesser than that of the Eearth, the astronauts certainly knew that their weight will be different on the Moon.
Learn more about weight:
https://brainly.com/question/1839641
In the harber-bosch process, the reactant nitrogen is drawn from the air while the hydrogen is produced by burning methane gas (CH4) in a series of processes that can be simplified as: CH4 + 2H2O —> CO2 + 4H2 3a. A small ammonia plant used 123,000 g of H2 gas per day. Determine the mass of CO2 (in g) that will be released as the H2 is produced. Show all work
Answers
Answer:Main Answer:
The mass of CO2 released in the production of 123,000 g of H2 gas is 265,200 g.
Explanation: From the given equation, we can see that the production of 4 moles of H2 requires the combustion of 1 mole of CH4, resulting in the release of 2 moles of H2O and 1 mole of CO2. The molar mass of H2 is 2 g/mol, and the molar mass of CO2 is 44 g/mol. Therefore, the mass of CO2 released in the production of 123,000 g of H2 is (123,000/2) x (1/4) x 44 = 265,200 g.
How many atoms are in 25.0 moles of calcium (Ca)?
Answers
Answer:
1.8066e+24 is the answer
Classify each property according to whether it is displayed by metals or by nonmetals
a. low melting point b. shiny dull c. poor conductor d. ductile e. malleable f. brittle g. good conductor h. high melting point
Answers
a. low melting point: Nonmetals. b. shiny: Metals. c. poor conductor: Nonmetals. d. ductile: Metals. e. malleable: Metals. f. brittle: Nonmetals. g. good conductor: Metals. h. high melting point: Metals
a. Low melting point is generally displayed by nonmetals. Metals tend to have high melting points.
b. Metals are typically shiny due to their ability to reflect light.
c. Nonmetals are generally poor conductors of heat and electricity.
d. Metals are ductile, meaning they can be drawn into thin wires without breaking.
e. Metals are malleable, meaning they can be hammered into thin sheets or shapes without shattering.
f. Nonmetals are usually brittle, meaning they are prone to breaking or shattering when subjected to stress.
g. Metals are good conductors of heat and electricity.
h. Metals typically have high melting points compared to nonmetals.
It's important to note that these are general trends and not absolute characteristics for all metals or nonmetals. Some exceptions or variations may exist within specific elements or compounds.
Learn more about properties of metals and nonmetals here: brainly.com/question/638020
#SPJ11
1. The characteristics of an acid are that it has a _____________ taste, reacts with _____________, and turns litmus paper into ______________.
2. When you add TOO MUCH solute to a solution, the solution becomes ____________________.
3. A mixture contains a ___________________ and a ___________________.
4. You test a liquid and its pH is 7. This liquid is _____________________.
5. Lemon juice, apple juice, and vinegar all have pH measurements below seven (7), making them a (n) __________.
6. Ammonia, blood, and drain cleaner all have pH measurements above seven (7), making them a (n) ________________.
7. ___________________ is another word for eating away at material.
8. __________ can be an acid or base because it is a good conductor of electricity.
9. __________ is a positive ion and __________ is a negative ion.
10. Two or more substances mixed but not chemically combined are a _____________.
Answers
Answer:
Explanation:
1. The characteristics of an acid are that it has a sour taste, reacts with metals, and turns blue litmus paper into red.
2. When you add TOO MUCH solute to a solution, the solution becomes saturated at some point. It will not dissolve anymore and will remain solid instead.
3. A mixture contains an acid and a base.
4. You test a liquid, and its pH is 7. This liquid is Neutral.
5. Lemon juice, apple juice, and Vinegar all have pH measurements below seven (7), making them acidic.
6. Ammonia, blood, and drain cleaner all have pH measurements above seven (7), making them basic.
7. Erosion is another word for eating away at material.
8. Vinegar can be an acid or base because it is a good conductor of electricity.
9. Cation is a positive ion and Anion is a negative ion.
10. Two or more substances mixed but not chemically combines are a Mixture.
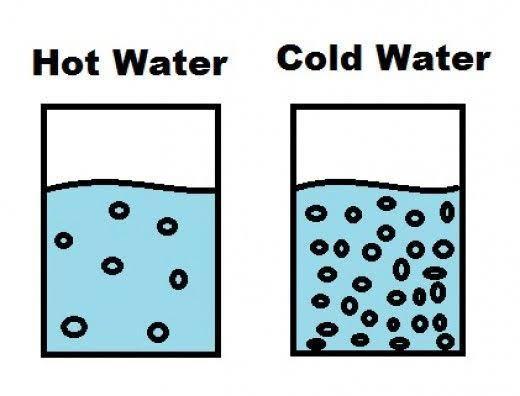
3- You saw a jar of hot water placed upside down over a jar of cold water. The hot
water stayed on top of the cold water without mixing. Why did the hot water stay on
top of the cold water?
Answers
Hot water stays on the top of cold water and doesn't mix. This is because of difference in DENSITY of water at different temperatures.
When we heat water, then the atoms gain energy from the heat and start moving away from each other. Now, when the atoms move away from each other than the (mass per unit volume) i.e. the density decreases. This makes water lighter.
When the temperature is less i.e. cold water then the atoms are more compactly packed and therefore the density of water is more at lower temperatures.
Since, hot water is lighter than cold water that is why it stays on top of the cold water.
according to the universal law of gravitation, every object attracts every other object in the universe. why cant you feel the force of attraction between you and mars?
Answers
We can't feel the force of attraction between us and mars due to the large distance and negligeable gravitation.
What is Gravitation ?Gravitation is the universal force of attraction acting between all matter.
According to the universal law of gravitation, the effect of the force of gravity decreases as the distance increases.
The gravitation force is inversely proportional to the radius or the distance.
So, the gravitational force exhibited by the mars is not experienced by us due to the large distance.
Learn more about gravity here ;
https://brainly.com/question/4014727
#SPJ1
Answer: D.
Mars is a long distance away.
Explanation: plato :3
Hope this helps!

VO2max (ml kg−1⋅min−1)=3.5+483/1.5mi time in minutes - Review GETP 10 page 86 1. Calculate the estimated VO2 Max for a 1.5 mile run/walk field test performed by a 27 year-old man with the following results: 1.5 mile run time: 13 minutes VO2 Max: ml⋅kg−1⋅min−1 Multi-Stage Estimation of VO2Max - b=(SM2−SM1)/(HR2−HR1) - VO2Max=SM2+b(HRmax−HR2) - SM=VO2 of submaximal workload [use ACSM equations] Revicw an example of this equation on page 102 in your Advanced Fitness textbook. 1. Calculate the estimated VO2 Max for a 40 -year old woman with the following VO2 and heart rate values for two stages of a submaximal test.
Stage 1:
Stage 2:
VO2: 24.5ml/kg/min
VO2: 35.7ml/kg/min
HR: 145bpm
HR: 162bpm
Power [kgm/min]= Force [kg]× Flywheel Distance × RPM "Note: Convert kgm/min to Watts by dividing by 6. Review an example of this equation on page 95 in your Advanced Fitness textbook. 1. Calculate the kgm/min and Watts produced when cycling against 4 kg of resistance with a 6 m/ revolution flywheel at a pedaling rate of 60rpm. Kgm/min : Watts: ACSM Cycling/Leg Ergometry VO2=(Work[kgm/min]/ Mass [kg]×1.8)+3.5+3.5 Review an example of this equation on page 97 in your Advanced Fitness textbook.
Answers
When cycling against 4 kg of resistance with a 6 m/revolution flywheel at a pedaling rate of 60rpm, the power produced is approximately 1440 kgm/min or 240 Watts.
VO2max refers to the maximum volume of oxygen that a person can consume per kilogram of body weight per minute. It is commonly used as a measure of cardiorespiratory fitness. The equation you provided is one method to estimate VO2max based on a 1.5-mile run/walk field test and heart rate data.
To calculate the estimated VO2max for the 27-year-old man who completed the 1.5-mile run in 13 minutes, we need to substitute the given values into the equation:
VO2max (ml kg−1⋅min−1) = 3.5 + 483 / (1.5 mi time in minutes)
Substituting the values:
VO2max = 3.5 + 483 / 13
Simplifying the equation:
VO2max = 3.5 + 37.15
Calculating the sum:
VO2max ≈ 40.65 ml kg−1⋅min−1
Therefore, the estimated VO2max for the 27-year-old man is approximately 40.65 ml kg−1⋅min−1.
Regarding the second question, we can use the given VO2 and heart rate values for two stages of a submaximal test to estimate the VO2max for the 40-year-old woman. To do this, we need to use the formula:
b=(SM2−SM1)/(HR2−HR1)
VO2Max=SM2+b(HRmax−HR2)
Let's calculate the values step by step:
Substituting the given values:
b = (35.7 - 24.5) / (162 - 145)
Calculating the value of b:
b = 11.2 / 17
Substituting the values into the second part of the equation:
VO2Max = 35.7 + (11.2 / 17) * (HRmax - HR2)
To proceed further, we need the value of HRmax. Since it's not given in the question, we cannot provide an accurate estimation for the VO2max of the 40-year-old woman. I apologize for any inconvenience.
For the third question, we can calculate the kgm/min and Watts produced when cycling against 4 kg of resistance with a 6 m/revolution flywheel at a pedaling rate of 60rpm.
To calculate kgm/min:
Power [kgm/min] = Force [kg] × Flywheel Distance × RPM
Power [kgm/min] = 4 kg × 6 m × 60 rpm
Simplifying the equation:
Power [kgm/min] = 1440 kgm/min
To convert kgm/min to Watts, we divide by 6:
Watts = Power [kgm/min] / 6
Watts = 1440 kgm/min / 6
Calculating the value:
Watts ≈ 240 W
Therefore, when cycling against 4 kg of resistance with a 6 m/revolution flywheel at a pedaling rate of 60rpm, the power produced is approximately 1440 kgm/min or 240 Watts.
To know more about resistance, visit:
https://brainly.com/question/33728800
#SPJ11
Which technology is shown in the photograph?
A. Negative controls
B. Biostimulation reaction
C. Gel electrophoresis
D. Polymerase chain reaction
Answers
Answer:
C
Explanation:
Apex