Think back to the hypothesis formulated during the warm-up. For the unknown samples tested at the end of the experiment, the flame color should be
A/ the same as a sample with the identical metal ion.
B/ different from a sample with the identical metal ion.
C/ the same or different from a sample with the identical metal ion, depending on the metal.
Answers
Answer:c
Explanation:
Answer:
The answer is A
Explanation:
I just did it
Related Questions
A liquid ester used to flavour food is believed to be impure. What would be the best way of testing its purity?
Answers
Answer:
Filter it
Explanation:
What is Na2Co3? How look like that's?
Answers
Sodium carbonate, often referred to as Na2CO3, is a chemical compound composed of atoms of sodium (Na), carbon (C) and oxygen (O).
It is also sometimes called washing soda or soda ash. At room temperature, sodium carbonate is a white, crystalline solid that is very soluble in water. According to the chemical formula of the sodium carbonate molecule, Na2CO3, each molecule consists of two sodium atoms (Na), one carbon atom (C) and three oxygen atoms (O). The atomic configuration in sodium carbonate is shown in the given diagram.
A trigonal planar arrangement is formed when the central carbon atom is bonded to three oxygen atoms. The structure of sodium carbonate is completed by two sodium atoms joined to oxygen atoms.
Learn more about Sodium carbonate, here:
https://brainly.com/question/31422792
#SPJ1

In which phase transition do molecules move directly from a state involving vibration of particles in a fixed position to a state involving random movement of high-speed particles?
evaporation
sublimation
freezing
deposition
Answers
would be your answer because it releases other cells out and causes the needy of cells to evaporate and sink.
sublimation is the phase transition do molecules move directly from a state involving vibration of particles in a fixed position to a state involving random movement of high-speed particles.
What is Sublimation?In the process of printing sublimation shirts, an image is first printed on a special sheet of paper before being transferred to a different material, typically polyester or a polyester blend. After that, heat is applied to the ink until it fuses with the fabric.
Sublimation shirt printing is more expensive than other procedures, but it produces shirts that stay longer and don't peel or crack over time.
he primary distinction between sublimation and heat transfer is that during sublimation, just the ink transfers to the substrate. A transfer layer is frequently also transferred to the material during the heat transfer procedure.
Therefore, sublimation is the phase transition do molecules move directly from a state involving vibration of particles in a fixed position to a state involving random movement of high-speed particles.
To learn more about Sublimation, refer to the link:
https://brainly.com/question/29304516
#SPJ7
Calculate the percent composition by mass of each element in Al(OH)3. Use at least three significant figures.
Al =
0=
H=
Answers
Answer:
nope nope nope nope nope nope nope nope
Answer:
Al= (27/78)x100
Al= 34.6%
O= (48/78)x100
O= 61.5%
H= (3/78)x100
H= 3.85%
_______ occurs when a body’s molecular wavelength sends vibrations to another body, resulting in the production of another sound wave
Answers
Answer:
Natural frequency
Explanation:
Answer:
Resonance
Explanation:
Robert was changing the oil in his truck. He dumped the used oil on the ground in his yard. He didn't know it, but Robert was hurting the environment because the oil-
HELP FAST
Answers
Answer:
goes deep into the ground and pollutes the groundwater.
Calculate the Molarity of NaOH in the
solution prepared by dissolving its 4 g
in enough water to form 250 ml of the
solution.
Answers
Explanation:
naoh=40g/mol
250ml=0.25dm°3
naoh in g/dm°3=16g/dm°3
naoh in mol/dm°3=16g\dm°3
________
40g/mol
=0.4mol,, is the answer
What is the correct name for N2O
Answers
Explanation:
Nitrous oxide
^_^^.^^.^
The article also mentions
hydrogen
gas. Scientists hope to learn about
gas giants
from compressing this gas.
Answers
Answer:
By size, Jupiter reigns supreme in our solar system. This gas giant has 11 times the diameter of Earth. Astronomers have spotted even larger gas giants, such as the exoplanet Kepler-7b, located 1,400 light years away from Earth.
In our solar system, Jupiter and Saturn are gas giants. These planets make Earth look tiny. The diameter of Jupiter is 11 times bigger than that of Earth, and Saturn’s is nine times bigger. Some people also include Uranus and Neptune in the gas giant category. They have a lot of hydrogen and helium in their atmospheres. But these planets also have water, methane and ammonia, and so NASA places them in their own group.
Astronomers have spotted gas giants outside our solar system. Like Jupiter and Saturn, they aren’t very dense. But they can be even bigger or hotter than our solar system’s gas giants.
Explanation:
Hope it helps
conclusion for polarity of liquid
Answers
Answer:
The polarity of a liquid refers to the separation of electric charge within the molecules of the liquid, resulting in a positive and negative end. Based on this, we can draw the following conclusion:
In conclusion, the polarity of a liquid is an important property that affects its behavior and interactions with other substances. Polar liquids have molecules with an uneven distribution of charge, resulting in positive and negative ends. This polarity influences various aspects, such as solubility, surface tension, and the ability to dissolve other polar substances. Additionally, polar liquids tend to exhibit stronger intermolecular forces, leading to higher boiling and melting points compared to nonpolar liquids. Understanding the polarity of a liquid is crucial for various fields, including chemistry, biology, and material science, as it helps explain and predict the behavior and properties of different substances in a wide range of applications.
PLEASE MARK AS BRAINLIESTAnswer:
The polarity of a liquid refers to the separation of electric charges within the molecule, resulting in a molecule with a positive end and a negative end. The presence or absence of polarity in a liquid has significant implications for its behavior and interactions with other substances.
In conclusion, the polarity of a liquid plays a crucial role in determining its physical and chemical properties. Polar liquids, such as water, have an unequal distribution of charge within their molecules, leading to hydrogen bonding and strong intermolecular forces. These interactions give rise to properties like high boiling points, surface tension, and solubility, making polar liquids excellent solvents and essential for many biological processes.
On the other hand, nonpolar liquids, such as hydrocarbons, have a symmetrical distribution of charge and lack strong intermolecular forces like hydrogen bonding. As a result, they have lower boiling points, weaker interactions, and are typically less soluble in polar solvents. Nonpolar liquids are commonly used as solvents for nonpolar compounds and have different applications in various industries.
Understanding the polarity of a liquid is crucial in fields such as chemistry, biology, and materials science. It helps predict how substances will interact and dissolve in a given solvent, as well as how they will behave in chemical reactions. Additionally, polarity affects the physical properties of liquids, including their viscosity, conductivity, and surface behavior.
In summary, the polarity of a liquid is a fundamental characteristic that influences its behavior, solubility, and reactivity. Whether a liquid is polar or nonpolar has far-reaching consequences in various scientific disciplines and practical applications
3.
Two elements R and S combine to form
the compound RS^2.Which of the following
statements is true?
A:An energy change occurs when RS2
is formed.
B :RS^2 will have a colour in between those of
R and S.
C:RS^2 have the properties of both
R and S.
D:The ratio of R:S atoms is 2:1.
Answers
Answer:
C:RS₂ have the properties of both R and S.
Explanation:
A compound formed between the two elements R and S to form RS₂ will have properties that reflects both R and S.
This is chemical change and the atoms combine will influence how the the product is formed.
Compounds are substances that are composed of two or more kinds of atoms joined together in a definite grouping.
In the nucleus, a sodium atom (Na) has 11p+ and 12 nºo. How would you write the
sodium ion based on the loss of one electron?
Answers
Answer:
Na⁺
Explanation:
Given parameters:
Number of protons = 11
Number of neutrons = 12
Writing the expression based on sodium ion that lost one electron;
In a neutral sodium atom, the number of protons and electrons are the same. Number of protons are the the positively charged particles in an atom.
Number of electrons are the negatively charged particles.
If an atom loses electron for instance sodium:
Number of electrons = 10
Number of protons = 11
Charge on the atom = 11 - 10 = 1+
So, the symbol of the atom is Na⁺
Helppppp pleaseeee xxxxxx
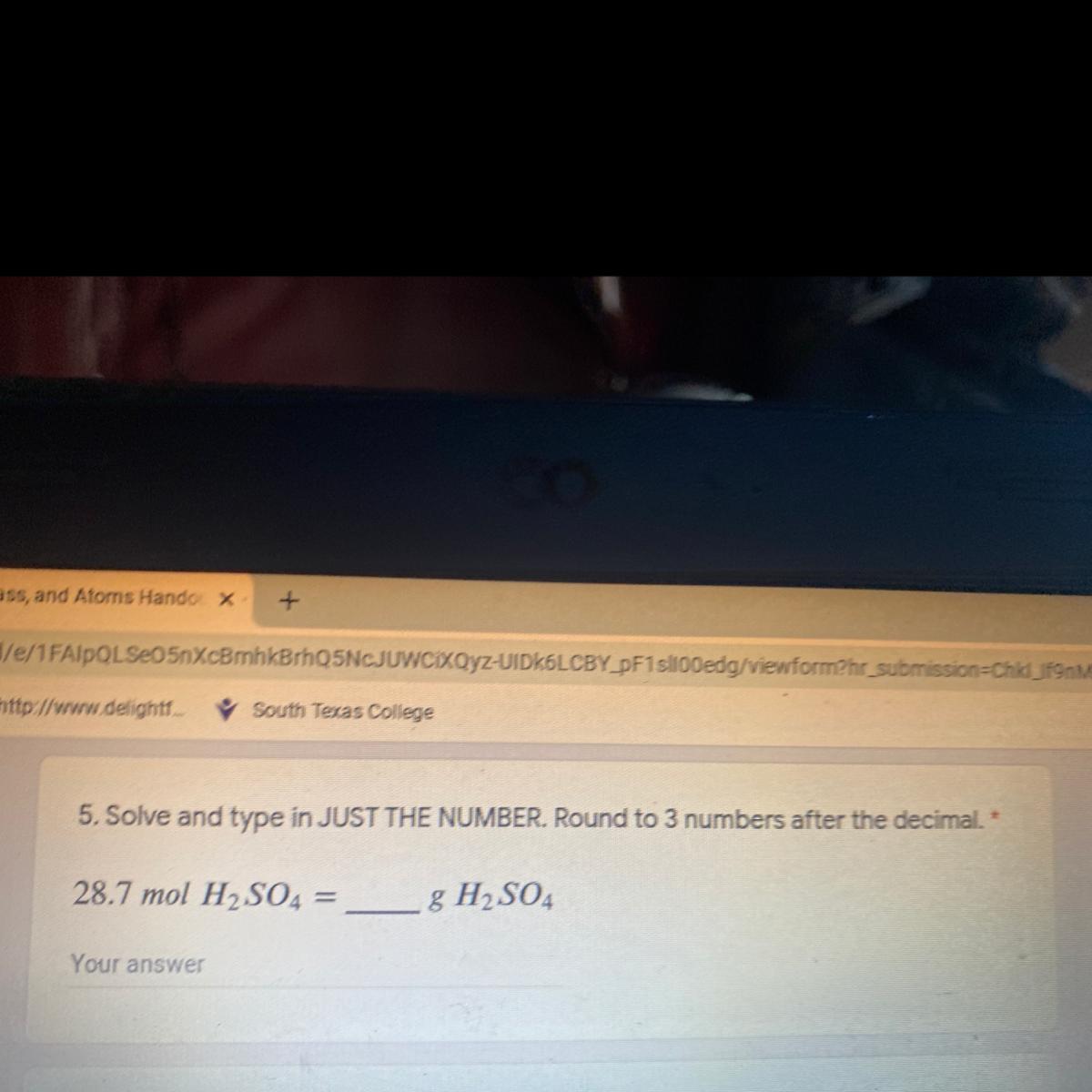
Answers
Answer:
2812.6 g of H₂SO₄
Explanation:
From the question given above, the following data were obtained:
Mole of H₂SO₄ = 28.7 moles
Mass of H₂SO₄ =?
Next, we shall determine the molar mass of H₂SO₄. This can be obtained as follow:
Molar mass of H₂SO₄ = (1×2) + 32 + (16×4)
= 2 + 32 + 64
= 98 g/mol
Finally, we shall determine the mass of H₂SO₄. This can be obtained as follow:
Mole of H₂SO₄ = 28.7 moles
Molar mass of H₂SO₄ =
Mass of H₂SO₄ =?
Mole = mass / Molar mass
28.7 = Mass of H₂SO₄ / 98
Cross multiply
Mass of H₂SO₄ = 28.7 × 98
Mass of H₂SO₄ = 2812.6 g
Thus, 28.7 mole of H₂SO₄ is equivalent to 2812.6 g of H₂SO₄
She collected 20 cm³ of oxygen. What volume of hydrogen could she also have collected at the same time?
Answers
Answer:
Assuming it was collected from the atmosphere it would be virtually nothing
Explanation:
hydrogen makes up 0.000055% of the atmosphere while oxygen makes up 23 percent. 20/400000 cm^3 of hydrogen
How many liters would you need to make a 1 m solution if you have 6 mol of sodium hydroxide.
Answers
The liters would we need to make the 1 M solution if we have 6 mol of sodium hydroxide of 6 L.
The moles of the sodium hydroxide = 6 mol
The molarity of the sodium hydroxide = 1 M
The expression for the molarity is as follows :
The molarity = moles / volume in L
The volume of the sodium hydroxide = moles / molarity
The volume of the sodium hydroxide = 6 / 1
The volume of the sodium hydroxide = 6 L.
Thus the volume of the sodium hydroxide is 6L in the 1 M of the solution.
To learn more about moles here
https://brainly.com/question/1097767
#SPJ4
Which statement BEST describes the strength of ionic and covalent bonds
Answers
Ionic bonds are the strongest type of chemical bond and, therefore, most compounds remain solid with very high melting points
The statement best describes the strength of ionic and covalent bonds is that ionic bond is the strongest bond and has very high melting point.
What are different types of bonding in chemistry?A chemical bond is a lasting attraction between atoms or ions that enables the formation of molecules, crystals, and other structures. The bond may result from the electrostatic force between oppositely charged ions as in ionic bonds, or through the sharing of electrons as in covalent bonds.
Types of Chemical Bonds includes-
Ionic Bonds.
Covalent Bonds.
Hydrogen Bonds.
Polar Bonds.
Generally, ionic bonds are much stronger than covalent bonds. In ionic bonds, there is complete transfer of electrons between elements to form a stable compound. While in covalent bond, there is only sharing of electrons between two elements to form a stable compound.
Therefore, The statement best describes the strength of ionic and covalent bonds is that ionic bond is the strongest bond and has very high melting point.
Learn more about chemical bonding here:
https://brainly.com/question/12907148
#SPJ2
1pt Which particle is a neutron most equal to in mass?
A. a molecule
B. an atom
O c. a proton
OD. an electron
Answers
Answer:
c. a proton
Explanation:
A neutron is most equal in mass to a proton.
A neutron is a subatomic particle without any charges on them.
A proton is a subatomic particle with a positive charge.
the mass of a proton and neutron are the most similar in an atom. the mass of a proton is 1.67 x 10⁻²⁷kgSo also is the mass of a neutronthe mass of an electron is 9.11 x 10⁻³¹kgWhat is the % mass/mass of a methanol solution prepared by mixing 70 grams of ethanol and 800 grams of water
Answers
Considering the definition of percentage by mass, the %mass/mass of a methanol solution prepared by mixing 70 grams of ethanol and 800 grams of water is 8.05 %.
Percentage by massThe percentage by mass expresses the concentration and indicates the amount of mass of solute present in 100 grams of solution.
In other words, the percentage by mass of a component of the solution is defined as the ratio of the mass of the solute to the mass of the solution, expressed as a percentage.
The percentage by mass is calculated as the mass of the solute divided by the mass of the solution, the result of which is multiplied by 100 to give a percentage. This is:
\(percentage by mass= \frac{mass of solute}{mass of solution}x100 \)
In this caseIn this case, you know:
mass of solute= 70 gmass of water= 800gmass of solution= mass of solute + mass of water= 70 g+ 800 g= 870 gReplacing in the definition of percentage by mass:
\(percentage by mass= \frac{70 g}{870 g}x100 \)
Solving:
percent by mass= 8.05 %
Finally, the %mass/mass of a methanol solution prepared by mixing 70 grams of ethanol and 800 grams of water is 8.05 %.
Learn more about percentage by mass:
brainly.com/question/19168984?referrer=searchResults
brainly.com/question/18646836?referrer=searchResults
is NO2 an ionic or covalent bond? Will give brainliest for the correct answer
Answers
Answer:
Covalent
Explanation:
hi, How are you?
NO2 is already polar, due to the fact that nitrogen shares a maximum of 3 electrons. Thus N makes two covalent bonds with one oxygen, one covalent and one dative with the other. And there's still a free electron left that causes the molecule to bend, forming an angle, and so the NO2 molecule is polar with an angle other than 180.
Describe how this instrument works.
Operation:

Answers
Answer:
Microscope
Explanation:
Answer:
its a telescope you see stars or plants
Explanation:
A sample of a certain lead compound contains 12.92 g of lead for 2 g of oxygen. A second sample has mass of 34.27 g and contains 14.39 g of oxygen. Are the two compound the same
Answers
The two chemical compounds are not the same, because their ratio is not equal. In both samples the composition of lead and oxygen is different.
What is a chemical compound?A chemical compound is a substance made of numerous similar molecules (or molecular entities) joined by chemical bonds and comprising atoms from various chemical elements. Therefore, a molecule made up of only one type of atom is not a compound. Chemical reactions, which may entail interactions with other molecules, can change a compound into a distinct substance. Atomic bonds may be broken or new ones created during this process.
What are the calculations?sample 1 = mass of lead / mass of oxygen = 12.92g/2g = 6.46 .
sample 2 = mass of lead/ mass of oxygen = 34.27 - 14.39/14.39 = 1.38 .
so, the ratios are not the same.
Hence, the two chemical compounds are not the same, because their ratio is not equal. In both samples the composition of lead and oxygen is different.
To know more about Chemical compounds, check out:
https://brainly.com/question/26487468
#SPJ1
Consider the reaction: 2HBr(g)->H2(g)+Br2(g)
a) Express the rate of the reaction in terms of the change in concentration of each of the reactants and products.
b)In the first 25.0s of this reaction, the concentration of HBr dropped from 0.600M to 0.512M. Calculate the average rate of the reaction.
c)If the volume of the reactino vessel in part b was 1.50L, what amount of Br2(in moles) was formed during the first 15.0s of the reaciton?
Answers
a) The rate of the reaction in terms of the change in concentration of each of the reactants and products will be: -d[HBr]/dt or -d[Br2]/dt or d[H2]/dt
b) The average rate of the reaction will be 0.00352 M/s
c) = 0.0352 M amount of Br2(in moles) was formed during the first 15.0s of the reaciton.
a) The rate of a reaction can be expressed as the change in concentration of a reactant or product over time.
For this reaction, the rate could be expressed as:
-d[HBr]/dt or -d[Br2]/dt or d[H2]/dt
b) To calculate the average rate of the reaction, we can use the formula:
average rate = (change in concentration of HBr) / (change in time)
where the change in time is 25.0 s and the change in concentration of HBr is 0.600 M - 0.512 M = 0.088 M.
average rate = 0.088 M / 25.0 s
= 0.00352 M/s
c) To calculate the amount of Br2 formed during the first 15.0s of the reaction, we need to multiply the average rate of the reaction by the change in time:
amount of Br2 = average rate * change in time
amount of Br2 = 0.00352 M/s * 15.0 s = 0.0528 moles
Since the volume of the reaction vessel is 1.50 L, we can find the concentration of Br2:
[Br2] = amount of Br2 / volume
[Br2] = 0.0528 moles / 1.50 L
= 0.0352 M
For more questions on Finding the concentration in Chemical reaction
https://brainly.com/question/19340344
#SPJ4
1) To increase the amount of NH3 at 200 atm, the manufacturer should (increase, decrease, not change) the temperature of the reaction chamber.
2) This change in temperature would shift the reaction to the (left, right) because this equilibrium reaction is (exothermic, endothermic)
Answers
The temperature of the reaction should be decreased
This change in temperature would make the equilibrium to shift to the right.
What is the LeChatelier principle?
The Le Chatelier's principle, commonly referred to as the Le Chatelier's principle of equilibrium, is a chemical principle that describes how an equilibrium system reacts to environmental changes.
According to this theory, when an equilibrium system is exposed to an outside force, it will respond in a way that partially offsets the imposed change and restore equilibrium.
Learn more about LeChatelier principle:https://brainly.com/question/31377984
#SPJ1
One of the most widely used isotopes in medical diagnostics is technetium-99m (the m indicates that it is a metastable isotope). Write the symbol for this isotope, indicating both mass number and atomic number. Express your answer as an isotope.
Answers
Atomic number of Technetium is 43, Mass number is 99 and symbol is Tc ,hence symbol of isotope is 99 m43 Tc
General representation of atom is AZ X
Where , A is mass number , Z is atomic number and X is symbol of element. Atomic number of Technetium is 43, Mass number is 99 and symbol is Tc ,hence symbol of isotope is 99 m43 Tc ,Where m stands for meta stable.
A chemical element with the atomic number 43 and the symbol Tc is known as technetium. It is the lightest element whose radioactive isotopes are all present. Technetium is a synthetic element that is created in abundance. The most prevalent source of naturally occurring technetium is uranium and thorium ores, where it spontaneously fissions as a byproduct, while molybdenum ores produce it as a byproduct of neutron capture. This crystalline, silvery-gray transition metal in group 7 of the periodic table is sandwiched between rhenium and manganese, and its chemical characteristics fall somewhere in the middle of the two nearby elements. Only in traces, 99Tc is the most prevalent naturally occurring isotope.
Learn more about Technetium here:
https://brainly.com/question/16184700
#SPJ4
moles of each product that would form as a result of the decomposition of aspirin
Answers
The decomposition of aspirin (acetylsalicylic acid,\(C_{9} H_{8} O_{4}\)) can occur through the hydrolysis reaction, resulting in the formation of acetic acid (\(CH_{3} COOH\)) and salicylic acid (\(C_{7} H_{6}O_{3}\)).
The decomposition of aspirin (acetylsalicylic acid, \(C_{9} H_{8} O_{4}\)) can occur through the hydrolysis reaction, resulting in the formation of acetic acid (\(CH_{3} COOH\)) and salicylic acid (\(C_{7} H_{6}O_{3}\)). To determine the moles of each product formed, we need to consider the balanced chemical equation for the reaction:
\(C_{9} H_{8} O_{4} = > C_{7} H_{6}O_{3} +CH_{3} COOH\)
From the equation, we can see that for every 1 mole of aspirin, 1 mole of salicylic acid and 1 mole of acetic acid are produced.
Therefore, the moles of salicylic acid and acetic acid formed will be equal to the number of moles of aspirin that decomposes. If we know the amount of aspirin in moles, we can directly calculate the moles of each product based on stoichiometry.
For more question on aspirin
https://brainly.com/question/25794846
#SPJ8
An argon ion laser emits visible radiation with photons of energy 4.071 x 10-19 J. What is the
wavelength of the radiation?
Answers
The wavelength of the radiation emitted by the argon ion laser is \(4.854 * 10^-7 m\).
Wavelength is a property of any type of wave that refers to the distance between two adjacent points on the wave that is in phase, i.e., at the same point in their respective cycles. It is usually denoted by the Greek letter lambda (λ) and is measured in units of length, such as meters or nanometers.
The energy carried by the photon (E) is related to the wavelength (\(\lambda\)) through the following equation:
\(E=hc/\lambda\); where 'h' is the Plank's Constant and 'c' is the speed of light which is \(3* 10^{-7} m/s\).
We can say that
\(\lambda - hc/E\)
Now after substituting the given values, we get:
\(\lambda = (6.626 * 10^{-34} J.s * 3.00 * 10^8 m/s) / (4.071 * 10^{-19} J)\\\lambda = 4.854 * 10^-7 m\)
Therefore the wavelength of the radiation emitted by the argon ion laser is \(4.854 * 10^-7 m\).
Learn more about the Plank's Constant at:
https://brainly.com/question/28060145
#SPJ4
Complete the equation for the equilibrium present in the region bc X(s)=?
Answers
The following equality statistic is available for the BC X (s) region states:
The following data is obtained using the equilibrium diagram:
shows the various categories that can be found in various temperatures and shapes.
It demonstrates how one object can solidly melt over another.
Describes the amount of the temperature at which the material system solidifies or solidifies.
The rate of reaction is equal to the rate of reaction in an equilibrium situation.
Examples of equilibrium include a book on the table, liquid in a tightly sealed container, full solution, ionic compounds in polar solvents, and the synthesis of ammonia.
Learn more about equilibrium here:
https://brainly.com/question/13463225
#SPJ9
If a snail crawls 55 inches per day, how many centimeters will he crawl in 23 days?
Group of answer choices
5.0X102 cm
1.06 cm
3.2X103 cm
6.1 cm
2.0X10-3 cm
Answers
If a snail crawls 55 inches per day ,the snail will crawl approximately \(3.2 * 10^3 centimeters.\)
To convert inches to centimeters, we need to use the conversion factor: 1 inch = 2.54 centimeters.
First, let's calculate the distance the snail crawls in inches in 23 days. We can multiply the daily distance by the number of days:
Distance in inches = 55 inches/day × 23 days = 1265 inches.
Now, to convert the distance from inches to centimeters, we multiply the distance in inches by the conversion factor:
Distance in centimeters = 1265 inches × 2.54 cm/inch = 3215.1 cm.
Rounding to the appropriate number of significant figures, the snail will crawl approximately 3215 cm or \(3.2 * 10^3 centimeters.\) in 23 days.
Therefore, the answer is Option 3:\(3.2 * 10^3 centimeters.\)
This means that in 23 days, the snail will crawl approximately\(3.2 * 10^3 centimeters.\)
Know more about centimeters here:
https://brainly.com/question/23883224
#SPJ8
15. What volume of water must be added to 300 mL of 0.75 M HCl to dilute the solution to
0.25 M?
Answers
Known :
V1 = 300 mL
M1 = 0.75 M
M2 = 0.25 M
Solution :
M1 • V1 = M2 • V2
(0.75 M) • (300 mL) = (0.25 M) V2
V2 = 900 mL
Water add to this solution is :
∆V = V2 - V1
∆V = 900 - 300
∆V = 600 mL
The answer is 600 ml of water needs to be added to change the concentration to 0.25 M.
What is Dilution ?A dilution is when you have a solution of a certain concentration and you add more solvent to decrease the concentration.
If you are adding more solvent, the volume of the whole solution is going to increase as the concentration of the solution decreases.
You can solve for the concentration or volume of the concentrated or dilute solution using the equation:
M₁V₁ = M₂V₂,
where M₁ is the concentration in molarity (moles/Liters) of the concentrated solution,
V₁ is the volume of the concentrated solution,
M₂ is the concentration in molarity of the dilute solution (after more solvent has been added), and
V₂ is the volume of the dilute solution.
It is given that
V₁ = 300 ml
V₂ = ?
M₁= 0.75 M
M₂ = 0.25 M
Substituting the values in the equation
0.75 * 300 = 0.25 * V₂
V₂= 900 ml
So 900 - 300 , 600 ml of water needs to be added to change the concentration to 0.25 M.
To know more about dilution
https://brainly.com/question/21323871
#SPJ2
What are five constitutional isomers of hydroxymethylcyclohexane that contain the cyclohexane ring?
Answers
The five constitutional isomers of hydroxymethylcyclohexane that contain the cyclohexane ring are:
hydroxymethylcyclohexanemethyl cyclohexanol2-methyl cyclohexanol3-methyl cyclohexanol4-methyl cyclohexanolHydroxymethylcyclohexane is an alcohol that contains a cyclohexane ring, as shown in the attached picture.
Constitutional or structural isomers are compounds with the same molecular formula but different structural formulas.
We can find the five constitutional isomers of hydroxymethylcyclohexane that contain the cyclohexane ring by attaching the hydroxyl group to the cyclohexane ring and varying the positions of the methyl group, as shown in the picture.
The five constitutional isomers of hydroxymethylcyclohexane that contain the cyclohexane ring are:
hydroxymethylcyclohexanemethyl cyclohexanol2-methyl cyclohexanol3-methyl cyclohexanol4-methyl cyclohexanolLearn more: https://brainly.com/question/14391080
