Answers
Answer:
The number of moles of AlBr3 = Molarity × Volume
= 0.400 × 0.700
= 0.28 moles
1 mole of AlBr3 gives 3 moles of Br- ions
That is
AlBr3 = Al3+ + 3Br-
Therefore, 0.045 moles of AlBr3 will yield 3 × 0.28 = 0.84 moles
Thus; they are 0.84 moles of bromide ions
Related Questions
how many moles of a solute is present in 4.00L of an 8.30M solution
Answers
Answer:
The number of moles of solute present in 4.00 L of an 8.30 M solution is 33.2
Explanation:
The Molarity (M) or Molar Concentration is the number of moles of solute per liter of solution; in other words it is the number of moles of solute that are dissolved in a given volume.
The molarity of a solution is calculated by dividing the moles of the solute by the volume of the solution:
\(Molarity=\frac{number of moles of solute}{volume}\)
Molarity is expressed in units (\(\frac{moles}{liter}\)) or M.
In this case:
molarity= 8.30 Mnumber of moles of solute= ?volume= 4.00 LReplacing:
\(8.30 M=\frac{number of moles of solute}{4 L}\)
Solving:
number of moles of solute= 8.30 M* 4 L= 8.30 \(\frac{moles}{liter}\) * 4 L
number of moles of solute =33.2
The number of moles of solute present in 4.00 L of an 8.30 M solution is 33.2
Answer:
33.2 is the answer
Explanation:
did the test already :)
Which process uses the sun's energy to convert water on Earth's surface into water vapor?
Condensation
Evaporation
Precipitation
Transpiration
Answers
Answer:
Evaporation
Explanation:
Feel free to give brainliest.
Have an amazing day!
Balance: _____H3(PO4)+ ____KOH-____K3(PO4)+_____ H2O
Answers
Answer: H3(PO4) + 3 KOH= K3(PO4)+ 3 H2O
Explanation: With these numbers everything is balanced on each side.
A balloon has a volume of 145 mL at room temperature (25°C = 298°K). Alyssa decides to place the balloon in the freezer to see what happens. After being in the freezer for an hour, the balloon has a new volume of 35mL. What is the temperature inside the freezer?
Answers
The temperature inside the freezer is approximately -164°C.
To solve this problem, we can use the combined gas law equation:
\((P1V1)/T1 = (P2V2)/T2\)
where P is the pressure, V is the volume, and T is the temperature of the gas.
We know that the initial volume of the balloon is 145 mL and the final volume is 35 mL. We also know that the initial temperature is 25°C or 298 K, and we need to find the final temperature.
Assuming the pressure of the gas remains constant, we can rearrange the combined gas law equation to solve for the final temperature:
\(T2 = (P2V2/T1)(P1/V1)\)
Plugging in the values we know, we get:
\(T2 = (1 atm * 35 mL/298 K)(1 atm/145 mL) = 0.0808 atm/mL\)
Multiplying both sides by 298 K and dividing by 0.0808 atm/mL, we get:
T2 = 109.15 K or approximately -164°C
Therefore, the temperature inside the freezer is approximately -164°C.
Learn more about combined gas law, here:
https://brainly.com/question/30458409
#SPJ1
How can kinetic energy of motion be transformed into other kinds of energy thermal energy

Answers
Answer:
Answer Below ↓
Explanation:
If the moving sand hits an obstacle, it stops due to the friction created by the contact and its kinetic energy is then transformed into thermal energy, or heat.
Magnesium has a density of 1.74 g/cm3. What is the volume of 58.6 g of Mg?

Answers
Answer:
1 kg = 1000 g
58.6=58,600g
Explanation:
Use dimensional analysis to solve the following problems. Pay attention to correct use of units and correct use of significant figures in calculations.
1) Convert 8.00 moles of aluminum to grams.
2) Convert 10.5 moles of aluminum chloride to grams.
Answers
Answer:
Solution given:
1:
1 mole of aluminum =26.98 gram
8 mole =26.98*8=215.84 gram
8.00 moles of aluminum =215.84grams.
2:
1 mole of aluminum chloride =133.34 grams
10.5 moles of aluminum chloride =133.34*10.5=1400.07 grams
10.5 moles of aluminum chloride =1400 grams.
#1
Molar mass of Al=27g/mol1mol.of Al=27g
8mol of Al=27(8)=216g
#2
Molar mass of AlCl3=133.5g/mol
1 mol of AlCl3=133.5g
10.5mol.of AlCl3=10.5(133.5)=1401.75g
I need help!!!!!!!!!!!!!!!!
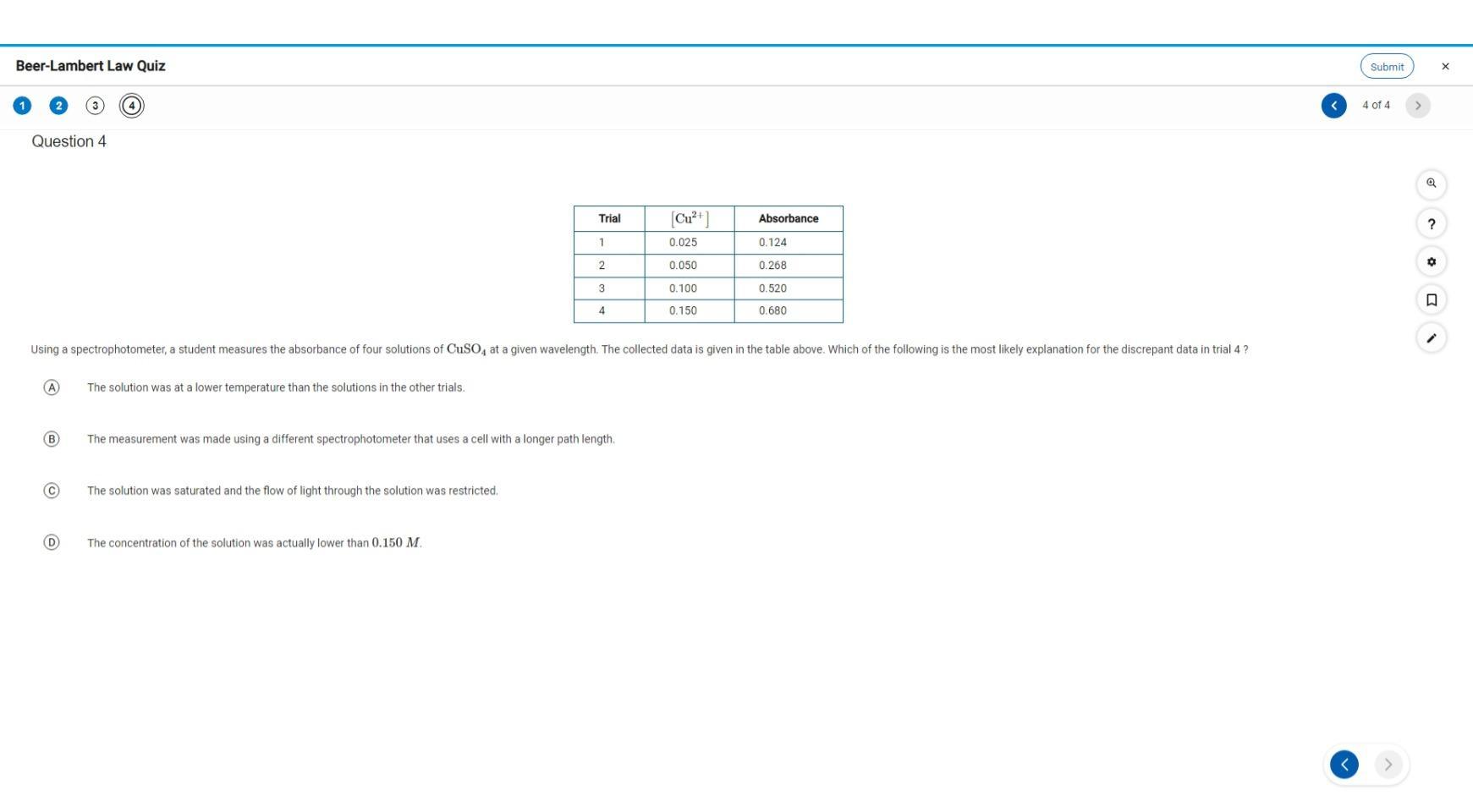
Answers
The reason for the fourth measurement is that the solution is saturated. Option C in the question.
What is the Beer Lambert's law?According to the Beer Lambert's law, the absorbance of the solution is found to the proportional to the concentration of the solution. This implies that as the concentration of the solution increases, the absorbance of the solution also increases.
The absorbance is proportional to the concentration, and the pathlength of the cell where the measurement is taken.
We can now see from the table that the absorbance of the solution is largely affected by the concentration of the solution and not really the pathlength.
Learn more about Beer Lambert's law:https://brainly.com/question/8831959
#SPJ1
Question 4
2 pts
669.0 mL of oxygen are collected over water at 17.0 °C and a total
pressure of 785.0 mm of mercury. What is the volume (in mL) of dry
oxygen at 60.0 °C and 847.0 mmHg pressure?
Question 5
2 pts

Answers
Answer:
711.96 mL
Explanation:
Using the combined gas law equation;
P1V1/T1 = P2V2/T2
Where;
P1 = initial pressure (mmHg)
P2 = final pressure (mmHg)
V1 = initial volume (mL)
V2 = final volume (mL)
T1 = initial temperature (K)
T2 = final temperature (K)
According to the information provided in this question,
P1 = 785.0 mmHg
P2 = 847.0 mmHg
V1 = 669.0 mL
V2 = ?
T1 = 17.0 °C = 17 + 273 = 290K
T2 = 60.0 °C = 60 + 273 = 333K
Using P1V1/T1 = P2V2/T2
785 × 669/290 = 847 × V2/333
525165/290 = 847 V2/333
1810.91 = 2.54 V2
V2 = 1810.91 ÷ 2.54
V2 = 711.96 mL
A compound with the empirical formula CH2
has a molar mass of 126
g/mol. What is the molecular formula for this compound?
Answers
C2H4 is the molecular formula. When we multiply the empirical formula by the empirical mass ratio, we get the molecular formula.
What do a compound and an element mean?Atoms from various elements are chemically mixed in a predetermined ratio to form a compound. A pure chemical compound known as an element is composed of only one type of atom. Composition. Different elements are found in compounds in fixed ratios organized in predetermined ways by chemical bonds.
How do elements and compounds differ from one another?A single sort of atom makes up the pure substances known as elements. Compounds are compounds made of multiple distinct kinds of elements combined chemically in predetermined ratios.
To know more about compound visit:
https://brainly.com/question/14117795
#SPJ1
which of the following concerning molecular geometry and dipole moments is correct? all molecules with polar bonds have a permanent dipole moment. all square planar molecules are nonpolar. all linear molecules are nonpolar. only molecules with polar bonds may have a permanent dipole moment. a molecule with nonpolar bonds could have a permanent dipole moment, depending on the molecular geometry.
Answers
Answer:
only molecules with polar bonds may have a permanent dipole moment.
Explanation:
how does work related to kinetic energy?
How are mass and velocity related in kinetic energy?
Answers
Answer:
Hope this would help u
How does work related to kinetic energy?
According to the work-energy theorem, the work done on an object by a net force equals the change in kinetic energy of the object. W = Δ K E Essentially kinetic energy is the energy used for motion. When things move, they can do work. As things move, they do work. that is what the above demonstrates ( W = Δ K E ). Work is the force on the object as it changes a distance. Interestingly, as work is done on an object, potential energy can be stored in that object. For example, if you carry a load up the stairs. Now that load will have potential energy that can be transformed into kinetic energy and so on. This where the Law of Conservation of Energy kicks in and provides the theory behind this praxis.How are mass and velocity related in kinetic energy?
The velocity would decrease because mass and velocity are inversely related. The equation for Kinetic Energy is: KE = 1/2 mv2. Kinetic energy has a direct relationship with mass, meaning that as mass increases so does the Kinetic Energy of an object.Explanation:
In the combustion of hydrogen gas, hydrogen reacts with oxygen from the air to form water vapor. hydrogen+oxygen⟶water
If you burn 46.2g of hydrogen and produce 413g of water, how much oxygen reacted?
mass of oxygen:
Answers
Answer:
ok, here is your answer
Explanation:
AI-generated answer
To find the mass of oxygen that reacted, we need to use the Law of Conservation of Mass, which states that in a chemical reaction, the mass of the reactants equals the mass of the products.
First, we need to find the number of moles of hydrogen that reacted:
Molar mass of hydrogen (H₂) = 2.016 g/mol
Number of moles of H₂ = mass/molar mass = 46.2 g/2.016 g/mol = 22.92 mol
Next, we need to use the balanced chemical equation to find the number of moles of water produced:
hydrogen + oxygen → water
2H₂ + O₂ → 2H₂O
From the equation, we can see that for every 2 moles of H₂, 1 mole of O₂ is required to produce 2 moles of H₂O. Therefore, the number of moles of O₂ required to produce 22.92 moles of H₂O is:
Number of moles of O₂ = 1/2 x 22.92 mol = 11.46 mol
Finally, we can find the mass of oxygen that reacted by using its molar mass:
Molar mass of oxygen (O₂) = 32.00 g/mol
Mass of oxygen = number of moles x molar mass = 11.46 mol x 32.00 g/mol = 366.72 g
Therefore, the mass of oxygen that reacted is 366.72 g.
mark me as brainliestWhich factor does not influence whether a substance will be a liquid at room temperature and normal atmospheric pressure?
O molecular mass
O surface tension
O molecular shape
O strength of intermolecular forces
20pts
Answers
Surface tension
Hope it helps
Which of the following is a correctly written thermochemical equation?
2C8H18 +25O2 → 16CO2 + 18H2O, ΔH = –5,471 kJ/mol
Correct Answer
C5H12 (g) + 8O2 (g) → 5CO2 (g) + 6H2O (l), ΔH = –3,536.1 kJ/mol
Incorrect Response
C3H8 (g) + O2 (g) → CO2 (g) + H2O (l), ΔH = –2,220 kJ/mol

Answers
Answer:
The second option
Explanation:
C5H12 (g) + 8O2 (g) → 5CO2 (g) + 6H2O (l), ΔH = –3,536.1 kJ/mol
Explanation:
The correctly written thermochemical equation should include the balanced chemical equation and the corresponding enthalpy change (ΔH). The balanced chemical equation must have equal numbers of atoms on both sides of the equation, and the state of each substance should be indicated. The enthalpy change should be written with the correct sign (positive or negative) and unit (kJ/mol).
The first equation is correctly balanced, but the enthalpy change is not correct. The correct enthalpy change for this reaction is –5,471 kJ/mol, but it is written as a positive value in the equation. The correct enthalpy change should be written as ΔH = –5,471 kJ/mol.
The second equation is correctly balanced and includes the correct enthalpy change (ΔH = –3,536.1 kJ/mol). Therefore, it is the correctly written thermochemical equation
List 3 pieces of information you would find on a periodic table or elements
Answers
?Are you good.....
sailing 2
a)at
b)in
Answers
Answer:
A
Explanation:
common sense
Answer:
are you good at sailing A.
7.5 L of a gas at 2 ATM and a temperature of 75°C is changed and volume to 3.4 L and a pressure of .5 ATM what is the new temperature
Answers
Answer:
Explanation:
Combined Gas Law
T2= T1P2V2/ (P1V1) = 348.15 X .5 X 3.4/(2 X 7.5) =39.46 K or -233.69C
Give and proidi the following after and undergoing alpha decay and beta decay
Answers
The products of the alpha decay of radium-226 and the beta decay of carbon-14 are radon-222 and nitrogen-14, respectively.
The alpha decay of radium-226 results in the emission of an alpha particle, which is a helium nucleus consisting of two protons and two neutrons.
Therefore, the product of the alpha decay of radium-226 is radon-222:
Ra-226 → Rn-222 + alpha particle
On the other hand, In the case of carbon-14, beta minus decay occurs, in which a neutron is converted into a proton, and an electron and an antineutrino are emitted.
So carbon-14 becomes nitrogen-14:
C-14 → N-14 + beta particle
To know more about alpha decay, here
brainly.com/question/27870937
#SPJ1
--The complete Question is, What is the product of the alpha decay of radium-226 and the beta decay of carbon-14?--
what was the open-range system
Answers
Answer: In the Western United States and Canada, open range is rangeland where cattle roam freely regardless of land ownership. ... Land in open range that is designated as part of a "herd district" reverses liabilities, requiring an animal's owner to fence it in or otherwise keep it on the person's own property.
Explanation: Mark me as brainliest
The element least likely to obey the octet rule in forming chemical bonds is
Answers
Answer:
The two elements that most commonly fail to complete an octet are boron and aluminum; they both readily form compounds in which they have six valence electrons, rather than the usual eight predicted by the octet rule.
I think it will help you.
describe how you can determine the total change in empathy and activation energy from the diagram and if each is positive or negative 
Answers
The difference between the products point on the reaction profile and the energy of the reactants is the reaction's activation energy. The change is negative
What is a diagram of potential energy?The energy change between the reactants and the products can be seen in a potential energy diagram or reaction profile.
When we examine the reaction profile, we see that the energy difference between the reactants and products indicates that the process is endothermic. Enthalpy is calculated by deducting the energy of the reactants from the energy of the products.
Learn more about energy profile: brainly.com/question/11256472
#SPJ1

Find the mass of 3.9 x 1023 molecules of carbon dioxide gas at STP conditions. *
O 28.6 grams
O 67.7 grams
O 76.4 grams
O 19.1 grams
Answers
The molar mass (formula weight) of carbon dioxide (CO2) is 44.01 g/mol.
At STP (Standard Temperature and Pressure), which is defined as 0°C (273.15 K) and 1 atm of pressure, 1 mole of any gas occupies 22.4 L of volume.
Now, we have 3.9 x 10^23 molecules of CO2:
- To find the number of moles, we divide the number of molecules by Avogadro's number:
n = N/Na = 3.9 x 10^23/6.022 x 10^23 = 0.648 moles
- To find the mass, we multiply the number of moles by the molar mass:
mass = n x M = 0.648 mol x 44.01 g/mol = 28.52 grams
Therefore, the mass of 3.9 x 10^23 molecules of carbon dioxide gas at STP is approximately 28.6 grams (option A).
Calculate the molality of a 5.51 M ethanol (C2H5OH) solution whose density is 0.9349 g/mL
Answers
Molality of C2H5OH is 1.1.27m.
What is Molality?
Molality is no.of moles present in One Kg solution .
Molality is represented by m
m= no.of moles/ weigt of solution in kg
Given is Molarity= 51.30M
molar mass of C2H5OH is 46g / mol , Density =0.9349g/ ml
Density=m/V
V = 107 ml
Molarity= no of moles/ Volume
51.5×46×107= x × 1000
weight of C2H5OH = 27.7g
molality = 27.7/1000××46
m= 1.27m
to learn more about Molality click https://brainly.com/question/20380670
#SPJ9
21. If you have 350 milliliters (mL) of a 500-milligramper liter (mg/L) solution of alum (aluminum sulfate),how much water would you have to add to bring theconcentration down to 100 mg/L? Assume that youare adding water that does not contain any alum (C2=0).1. 70 mL2. 1,400 mL3. 143 mL4. 1,750 mL
Answers
1750mL
Explanations:In order to get the required volume, we will use the dilution formula expressed as:
\(C_1V_1=C_2V_2\)where:
C₁ and C₂ are the initial and final concentration respectively
V₁ and V₂ are the initial and final volume respectively
Given the following parameters:
C₁ = 500mg/L
C₂ = 100mg/L
V₁= 350mL
Required
V₂
Substitute the given parameters into the formula to have:
\(\begin{gathered} V__2=\frac{C_1V_1}{C_2} \\ V_2=\frac{500\times350}{100} \\ V_2=\frac{175,000}{100} \\ V_2=1750mL \end{gathered}\)Hence the amount of water needed is 1750mL
When an atom goes through alpha decay,
a. Only the atomic number changes
b. Only the mass number changes
c. Both the mass and atomic numbers change
d. Neither the mass nor atomic number changes, as only energy is emitted.
Answers
Urea [(NH2)2CO] is a by-product of protein metabolism, and it can be synthesized in a lab by combining ammonia and carbon dioxide according to the following equation 2NH3(g) + CO2(g) (NH2)2CO(aq) + H2O(l) determine the moles of carbon dioxide required to reaction with 2.50 moles of ammonia.
Answers
1.25 moles of \(CO_{2}\) are required to react with 2.50 moles of \(NH_{3}\).
What are the moles?
Moles are a unit of measurement used in chemistry to express the amount of a substance. One mole of a substance is equal to its molecular weight expressed in grams. In other words, one mole of a substance contains 6.022 x \(10^{23}\) individual particles (atoms, molecules, or ions). The number of moles of a substance can be calculated by dividing its mass in grams by its molecular weight or by using the ideal gas law PV=nRT, where n is the number of moles.
The balanced chemical equation is:
\(2NH_{3}\)(g) + \(CO_{2}\)(g) → \((NH_{2})_{2}CO\)(aq) + \(H_{2}O\)(l)
From the balanced equation, we can see that 1 mole of \(CO_{2}\) reacts with 2 moles of \(NH_{3}\).
Therefore, to determine the moles of \(CO_{2}\) required to react with 2.50 moles of \(NH_{3}\), we need to use the mole ratio from the balanced equation:
2 \(NH_{3}\) : 1 \(CO_{2}\)
2.50 moles \(NH_{3}\) * (1 mole \(CO_{2}\) / 2 moles \(NH_{3}\)) = 1.25 moles \(CO_{2}\)
Therefore, 1.25 moles of \(CO_{2}\) are required to react with 2.50 moles of \(NH_{3}\).
What is molecular weight ?
Molecular weight, also known as molar mass, is the mass of one mole of a substance. It is expressed in units of grams per mole (g/mol). The molecular weight of a compound is determined by adding up the atomic weights of all the atoms in the molecule.
What is substance?
In chemistry, a substance is a form of matter that has constant chemical composition and characteristic properties. It may be an element or a compound made up of two or more elements chemically combined in a fixed proportion. Examples of substances include pure water, carbon dioxide gas, and sodium chloride (table salt).
To know more about moles, visit:
https://brainly.com/question/13623076
#SPJ1
Below is a symbol block from the periodic table. What does the number 6 indicate?
Answers
Answer:
Carbon And the number of protons
Does anyone have Personal Care services on E2020
Answers
Here are some questions on Personal Care services on E2020 are:
A client with a new ileostomy has been home for four days. The HHA is giving the client a bath and notices that the pouch is full. The HHA should say to the client: D. "I'll empty the pouch for you."A client is bedridden at home and has an infected draining sacral wound. The infection control supplies that should be kept in the home is: Gloves.What is infection?An infection is the entrance and growth of dangerous microorganisms in the body that harm the host, such as bacteria, viruses, fungus, or parasites.
Infections can be systemic (affecting the entire body) or localized (affecting a particular area of the body), and they can be moderate to severe.
Learn more about infection on https://brainly.com/question/14083398
#SPJ1
If you have 50 grams of H2SO4 and excess
Al, how many grams of Al2(SO4)3 are
produced during the following reaction?
2 Al + 3 H2SO4 → Al2(SO4)3 + 3 H2
Answers
0.65mol \(Alx_{2} (So_{4} )_{3}\) are produced during the reaction.
What is the molar mass?The mass in grams of one mole of a chemical is its molar mass. A mole is the measurement of the number of things, such as atoms, molecules, and ions, that are present in a substance. Any substance's mole is. Molecules, 6.022 10 23.Divide each element's atomic weight (found in the periodic table) by the number of that element's atoms in the compound. 3. Add up the totals, and then follow the number with the units of grams/mole.The mass of every atom in a molecule, expressed in grams per mole, is known as the molar mass. We first obtain the atomic weights of the different elements in a periodic table to compute a molecule's molar mass. Then, we multiply the total number of atoms by each one's atomic mass.How many grams of Al2(SO4)3 are produced during the following reaction?
2 Al + 3 H2SO4 → Al2(SO4)3 + 3 H2
Molar mas:26.98 →341.15g/mol
All take 35g.
Moles of aluminum=35/26.98=1.29mol.
2mole of aluminum will give=1/2*1.29=0.65
Therefore, 0.65mol \(Alx_{2} (So_{4} )_{3}\).
To learn more about Molar mass, refer to:
https://brainly.com/question/837939
#SPJ9