The temperature of a sample of gas is 350K at 2.5 atm and 45.0 L. What is the new volume at standard temperature and pressure?
Answers
Answer:
The answer for V2 is 144 to the nearest whole number
Explanation:
P1V1/T1 = P2V2/T2.
P1=2.5atm
V1=45L
T1=350K
P2=1 atm at standard pressure
V2=?
T2=273 at standard temperature
P1V1/T1 = P2V2/T2.
V2=P1V1T2/P2T1
V2=2.5×45×350/1×273
V2=144.23
V2=144 to the nearest whole number
Related Questions
Typically, when two atoms form a chemical bond, the expected result is that the electrons.
Answers
When atoms form chemical bonding then the atoms attain Noble gas configuration.
Noble gas configuration of an atom includes the fundamental image of the ultimate noble fueloline previous to that atom, observed via way of means of the configuration of the ultimate electrons.so for sodium, we make the substitution of [Ne] for the 1s22s22p6 a part of the configuration. Sodium's noble fueloline configuration becomes [Ne]3s1.
Covalent bonds, atoms percentage pairs of electrons, at the same time as in ionic bonds, electrons are absolutely transferred among atoms in order that ions are formed.During the formation of a chemical bond, the predicted end result is that the electrons will whole a noble fueloline configuration in each atoms. Typically, while atoms shape a chemical bond, the predicted end result is that the electrons.
Read more about atom:
https://brainly.com/question/621740
#SPJ4
PLz help mee I really need the answer

Answers
Answer:
sodium ,Na and magnesium,Mg
A student wants to develop a model that categorizes various plants and animals as either heterotrophs or autotrophs. Which statement provides the BEST criteria for distinguishing which category the various
organisms should be placed within the model?
A
Heterotrophs are multicellular organisms that reproduce sexually, autotrophs are unicellular organisms that reproduce asexually.
B
Heterotrophs can produce their own food from inorganic sources such as carbon dioxide, autotrophs need to consume other organisms in the food chain for sustenance
C
Heterotrophs need to consume other organisms in the food chain for sustenance; autotrophs can produce their own food from inorganic sources such as carbon dioxide
D
Autotrophs are multicellular organisms that reproduce sexually; heterotrophs are unicellular organisms that reproduce asexually.
Answers
Classify each organic compound based on the functional group it contains
Answers
Organic compound which has -OH as functional group is classified as alcohols while those with -COOH as functional group are classified as carboxylic acids.
What is functional group?Functional group is defined as a substituent or group of atoms or an atom which causes chemical reactions.Each functional group will react similarly regardless to the parent carbon chain to which it is attached.This helps in prediction of chemical reactions.
The reactivity of functional group can be enhanced by making modifications in the functional group .Atoms present in functional groups are linked to each other by means of covalent bonds.They are named along with organic compounds according to IUPAC nomenclature.
Learn more about functional groups,here:
https://brainly.com/question/14618322
#SPJ1
answer the question.........
mb wrg subject but its biology

Answers
The description of nonpoint source pollution is dirty drainage from city roadways.
The correct option is A.
What is pollution?Pollution describes the presence of substances in the environment that are harmful to the living organisms present in that environment.
Pollution may be classified based on the source of the pollution into:
point source pollution - point source pollution refers to pollution whose origin or source point is easily identifiable such as sewage from homes and industries or smoke from industriesnon-point source pollution - non-point source pollution refers to pollution whose source point is not easily identifiable, rather, it occurs as a result of the runoff or water snow that then carries pollutants from various sources as they flow. For example, after a flood, the flood water carries several pollutants with it as it flows over drainages and the ground.Learn more about nonpoint source pollution at: https://brainly.com/question/1557306
#SPJ1
In a solution, what term is given to the liquid? a.Solute
b.Solvent
c.Reagent
Answers
Answer:
B solvent
Explanation:
corn oil has a density of 0.88 g/ml. what is the mass of 44.32 ml of corn oil? the mass of the corn oil is
Answers
The mass of the corn oil is 39.002 g.
Define density.
The ratio of mass to volume forms the compound measure known as density. The amount of matter per unit volume is measured by density.
Given:
Density of corn oil = 0.88 g/ml
Volume of corn oil = 44.32 ml
Mass= ?
To calculate the mass, density or volume of an object, we use the formula:
Mass = density * volume
where M is the mass,
D is the density, and
V is the volume of an object.
By substituting the values in the above formula, we get
Mass = density * volume
Mass
= 0.88 g/ml* 44.32 ml
= 39.002 g
Therefore, the mass of the corn oil is 39.002 g.
To learn more about density from the given link.
https://brainly.com/question/1354972
#SPJ4
How many moles of iron(Il) oxide are produced from
3.0 moles of oxygen and excess Iron Sulfide as described by the chemical equation below?
4FeS + 502 - 2Fe,03 + 4502
Answers
Answer:
6 moles of Iron(II) Oxide
Answer:
1.2 mol
Explanation:
What will most likely happen when stress is applied to an equilibrium reaction?
A) The system will not respond to the stress.
B) The system will use catalysts to change its equilibrium.
C) The equilibrium position will shift to increase the applied stress.
D) The system will change its concentration to shift to a new equilibrium position.
Answers
Answer:
option D is the correct answer of this question.
When stress is applied to an equilibrium reaction then the system will change its concentration to shift to a new equilibrium position.
So, the correct option is D.
What is equilibrium ?The stage at which the rate of formation of reaction is equal to rate of backward reaction in an reversible reaction is called equilibrium.
The reaction in such stage occur is called equilibrium reaction.
Factors affecting equilibrium reaction.There are some factors which affects the equilibrium.
Pressure : On increasing pressure of the gas present in reactant side shift the reaction to forward direction.Concentration : As concentration of the reactant increases then the reaction shifts in forward direction and as concentration of product is increases then the reaction moves in backward directionTemperatureNumber of moles.learn about equilibrium
https://brainly.com/question/13463225
#SPJ2
if you need some points and are good at science help!

Answers
Answer:
region 2 and region 3
Explanation:
you can tell by the color of the land my friends^^
Answer:
2, 3 They are closest to the equator, and the equator marks to hottest places on earth.
Explanation:
I hope this helps you.
Determine the number of moles of 725.0 g of BaSO4
Answers
Answer:
It would be 3.1063937724731447 moles.
Hope this helps :)
someone please help me asap please dont scroll
what type of relationship is each one showing, the 3 options are positive, negative or no relationship
please help

Answers
Answer:
1. positive
2. no relationship
3. negative
Explanation:
1. increasing would mean x and y are becoming greater
2. straight across would not be increasing x or y
3. decreasing would mean y (probably not x) is getting smaller
i hope that's what you're looking for
The pH of a solution in which OH negative concentration equals six. 9×10 to the -10 molarity
Answers
Answer:
The pH of a solution in which the concentration of hydroxide ions is 6.9 x 10^-10 M is approximately 4.84.
Explanation:
The pH of a solution can be calculated from the concentration of hydroxide ions (OH-) using the relationship between pH, pOH, and the ion product constant for water (Kw). The pOH of a solution is defined as the negative logarithm (base 10) of the hydroxide ion concentration: pOH = -log[OH-]. The pH and pOH of a solution are related by the equation pH + pOH = 14. The ion product constant for water at 25°C is Kw = [H+][OH-] = 1.0 x 10^-14.
In this case, the concentration of hydroxide ions is given as [OH-] = 6.9 x 10^-10 M. We can use this value to calculate the pOH of the solution:
pOH = -log[OH-] = -log(6.9 x 10^-10) = 9.16
Then, we can use the relationship between pH and pOH to calculate the pH of the solution:
pH + pOH = 14
pH + 9.16 = 14
pH = 14 - 9.16
pH = 4.84
Therefore, the pH of a solution in which the concentration of hydroxide ions is 6.9 x 10^-10 M is approximately 4.84.
Answer:
pH = 4.84
Explanation:
To find the pH of a solution, we can use the formula:
pOH = -log[OH-]
Given:
[OH-] = 6.9 × 10^-10 M
First, let's calculate the pOH of the solution:
pOH = -log(6.9 × 10^-10)
pOH ≈ -(-9.16)
pOH ≈ 9.16
Now, to find the pH, we can use the relation:
pH + pOH = 14
pH = 14 - pOH
pH = 14 - 9.16
pH ≈ 4.84
Therefore, the pH of the solution is approximately 4.84.
Please hurry i'm being timed!!! will make brainist!!!
explain the difference in the work performed by an environmental scientist and a civil engineer during the development of a geothermal site.
Answers
Environmental scientists prioritize environmental impact assessment, mitigation, and sustainability, while civil engineers focus on engineering and construction aspects of the geothermal site.
The difference in the work performed by an environmental scientist and a civil engineer during the development of a geothermal site is as follows:
1. Environmental Scientist: Their primary role is to assess and monitor the environmental impacts of the geothermal site development. They study the site's natural resources, flora, fauna, and potential environmental risks. Their work includes collecting data, analyzing potential impacts on the environment, and ensuring compliance with environmental regulations.
2. Civil Engineer: Their main responsibility is to design, plan, and oversee the construction of the geothermal site's infrastructure. This includes the development of roads, pipelines, and buildings necessary for the site's operation. They also work on the structural aspects of the project, ensuring that the design is safe, efficient, and sustainable.
In summary, an environmental scientist focuses on the environmental aspects and impacts of the geothermal site development, while a civil engineer focuses on the design and construction of the infrastructure. Both professionals work together to ensure the successful and sustainable development of the geothermal site.
You can learn more about the geothermal site at: https://brainly.com/question/21057712
#SPJ11
some invertebrates combine morphological simplicity with features of great structural or biochemical complexity. select all correct examples of this statement.
Answers
Atoms strive for a helium nucleus electron configuration, which consists of eight valence electrons, in accordance with the octet rule.
Simply put, what does biochemical mean?The research of chemical reactions that take place both in and out of living organisms is known as biochemistry. It is a research facility science that combines chemistry and biology. Biochemists can understand and address biological issues by utilizing their understanding of and proficiency with chemical processes.
What's an example of biochemistry?One such biological molecule is glucose. The Greek word for "life" is where the word bio- comes from. Any carbon-based substance found in living beings is referred to as a biochemical compound. The cells in the body of all living organisms are composed of biochemical substances.
To know more about biochemical visit:
https://brainly.com/question/11582799
#SPJ4
Which choice is not true of a liquid in a glass capillary with a convex meniscus?a. The liquid has strong cohesive forces.b. The liquid level will be lower inside the capillary when a capillary is inserted into a bowl of the liquid.c. The liquid will have a convex meniscus as it moves in the capillary.d. The behavior of the liquid is driven by strong interactions with the capillary glass.
Answers
The correct answer is the liquid level will be lower inside the capillary when a capillary is inserted into a bowl of the liquid.
When a liquid is placed in a glass capillary with a convex meniscus, the behavior of the liquid is driven by strong cohesive forces between the liquid molecules and strong interactions with the capillary glass. As a result, the liquid will have a convex meniscus, meaning that the surface of the liquid will be curved outward. This is due to the forces acting on the liquid in the capillary. However, when a capillary is inserted into a bowl of the liquid, the liquid level inside the capillary will be higher than the liquid level outside the capillary. This is because the cohesive forces between the liquid molecules are stronger inside the capillary, where the liquid is in contact with the walls of the capillary, than they are outside the capillary. As a result, the liquid will be pulled up the walls of the capillary, causing the liquid level inside the capillary to be higher than the liquid level outside the capillary. This is opposite to what is stated in choice b, which is why it is the correct answer.
to know more about meniscus-
https://brainly.com/question/28013459
#SPJ4
At what temperature does water freeze?
Answers
Answer:
32 degrees
Explanation:
how many grams of n2 are required to completely react with 3.03 grams of h2 for the following balanced chemical equation? A. 1.00 B. 6.00 C. 14.0 D. 28.0
Answers
The grams of N2 are required to completely react with 3.03 grams of H2 for the following balanced chemical equation is 14 g.
We may calculate the number of moles of H2 that will be used by dividing the amount of H2 that will be utilised by its molar mass. We may multiply that number by the molar mass of N2 to get how many grammes we should use. We can divide that mole quantity by 3 to determine how many moles of N2 the reaction will consume.
In the reaction 1 mole of N2 react with 3 mole of H2 and give 2 mole of NH3
mass of H2 = 3.03g
No of moles of H2 = 3.03g/2 gmol-1
= 1.51 mole
1.51 mole of H2 require N2 = (1/3)× 1.51 moles
= 0.50 mole N2
molar mass of N2 =28g/mol
Mass of N2 require = 0.50mole ×28g/mol
= 14g
Mass of N2 require = 14g.
Learn more about Number of grams:
https://brainly.com/question/28902645
#SPJ4
The answer is C. 14.0 grams of N2 are required to completely react with 3.03 grams of H2.
The balanced chemical equation is:
N2 + 3H2 -> 2NH3
From the equation, we can see that 1 mole of N2 reacts with 3 moles of H2 to produce 2 moles of NH3.
To find out how many grams of N2 are required to react with 3.03 grams of H2, we first need to convert 3.03 grams of H2 to moles:
moles of H2 = mass of H2 / molar mass of H2
moles of H2 = 3.03 / 2.016
moles of H2 = 1.505
Now, we can use the mole ratio from the balanced equation to find out how many moles of N2 are required to react with 1.505 moles of H2:
moles of N2 = (1.505 mol H2) / (3 mol H2/1 mol N2)
moles of N2 = 0.5017
Finally, we can convert moles of N2 to grams of N2:
mass of N2 = moles of N2 x molar mass of N2
mass of N2 = 0.5017 x 28.02
mass of N2 = 14.04
To learn more about balanced chemical equation click here
brainly.com/question/28294176
#SPJ11
What is the difference between compound and molecule with example?
Answers
A compound is a substance made up of two or more different elements that are chemically bonded together. A molecule, on the other hand, is the smallest unit of a chemical compound that has the chemical properties of that compound. In other words, a compound is a type of molecule.
For example, water (H2O) is a compound made up of two hydrogen atoms and one oxygen atom. The smallest unit of water that still has the chemical properties of water is a molecule of water, which is made up of two hydrogen atoms and one oxygen atom chemically bonded together.
Another example is table salt (NaCl), which is a compound made up of one sodium atom and one chlorine atom chemically bonded together. The smallest unit of table salt that still has the chemical properties of table salt is a molecule of table salt, which is made up of one sodium atom and one chlorine atom chemically bonded together.
It's worth noting that not all compounds are made up of molecules. For example, compounds like metals, which are made up of metal atoms packed closely together, do not have distinct molecules, because the atoms are not chemically bonded together in a specific ratio, but instead are held together by metallic bonds.
In summary, a compound is a substance made up of two or more different elements that are chemically bonded together, and a molecule is the smallest unit of a chemical compound that has the chemical properties of that compound. Examples of compounds include water and table salt, which can be broken down into their respective molecules, H2O and NaCl respectively.
Here you can learn more about compound and molecule
https://brainly.com/question/27794850#
#SPJ11
the pka of acetic acid is 4.76. what is the ratio of [ch3cooh] to [ch3coo−] at ph = 4.76?
Answers
The ratio of [CH₃COOH] to [CH₃COO⁻] is 1:1 at pH 4.76, meaning that equal amounts of acetic acid and its conjugate base are present in the solution.
To find the ratio of [CH₃COOH] to [CH₃COO⁻] at pH = 4.76, we can use the Henderson-Hasselbalch equation, which is:
pH = pKa + log ([A⁻]/[HA])
In this case, the pKa of acetic acid is 4.76, and the pH is also 4.76. Plugging these values into the equation, we get:
4.76 = 4.76 + log ([CH₃COO⁻]/[CH₃COOH])
Since both sides of the equation are equal, it means the logarithmic term must be equal to 0:
0 = log ([CH₃COO⁻]/[CH₃COOH])
To find the ratio, we can take the antilog of both sides:
1 = [CH₃COO⁻]/[CH₃COOH]
This indicates that the ratio of [CH₃COOH] to [CH₃COO⁻] is 1:1 at pH 4.76, meaning that equal amounts of acetic acid and its conjugate base are present in the solution. This pH value represents the buffering capacity of the acetic acid/acetate system, as the solution can effectively resist changes in pH when small amounts of acids or bases are added.
Learn more about Henderson-Hasselbalch equation here:
https://brainly.com/question/31495136
#SPJ11
Read the temperatures shown to the nearest 0.5°C.
I’ll mark you as brainlister

Answers
Answer:
The temperature to the nearest 0.5°C is 98.5°C
what may happen to eli and his father? check all that apply
Answers
They will not have to pay any cost because the accident was not Dave’s fault, Eli will be covered by Dave’s family car insurance policy, they will be protected by the other driver’s insurance. (B,C,D)
Explanation: just answered it and it was correct.
B. They will not have to pay any costs because the accident was not Dave’s fault.
C. Eli will be covered by Dave’s family car insurance policy.
D. They will be protected by the other driver’s insurance.
May I have brainliest please? :)

Information gathered from observations and
experimentation is called?
Answers
Answer: Observing
Explanation: Information gathered in an experiment is called data, and its represents observations derived from the methods of the experiment on its sample elements. Hope this helped! :)
The Information gathered from observations and experimentation is called empirical evidence. Empirical evidence is a significant part of scientific research and helps to further results and discussions.
What is scientific research?Scientific research is a well designed and organised steps to conducts a scientific experiment for solving a problem under study. The research methodologies include logical, economic and creative ways to reveal the solution for a problem.
Information obtained through testing or observation is known as empirical evidence. These data are logged and examined by scientists. The procedure, which is a key component of the scientific method, results in the confirmation or rejection of a hypothesis and, as a result, improves our knowledge of the universe.
A hypothesis can be tested and accurately evaluated using different types of data collection, such as experiments that aim to produce a quantifiable or observable reaction, trials that replicate experiments to test their effectiveness or other methods of data collection.
To find more on empirical evidence, refer here:
https://brainly.com/question/21483139
#SPJ2
The Keq for the interconversion for the two chair conformers of methylcyclohexane at 25 °C is 18. What % of the chair conformers have an axial methyl group?
A) 95 B) 75 C) 50 D) 25 E) 5
Answers
The Keq for the interconversion for the two chair conformers of methylcyclohexane at 25 °C is 18. The % of the chair conformers having an axial methyl group is A) 95%.
If the value of Keq is higher than 1, that means the reaction is favored in the forward direction. If the value of Keq is lower than 1, that means the reaction is favored in the reverse direction.If the value of Keq is equal to 1, that means the reaction is at equilibrium.Methylcyclohexane is an example of the cyclohexane molecule in which there is a substitution of a methyl group on one of the six carbon atoms in the ring structure. The two chair conformers of methylcyclohexane are the axial and equatorial conformers, in which the methyl group is either in an axial or equatorial position, respectively.
The percentage of chair conformers having an axial methyl group can be calculated using the following formula:
% axial conformer = Keq / (1+Keq) × 100
Putting the value of Keq in the formula:
axial conformer = 18 / (1+18) × 100%
axial conformer = 18/19 × 100%
axial conformer = 94.7%
The value of percentage has been rounded off to the nearest whole number, which is 95. Therefore, the answer is (A) 95.
To learn more about equatorial position check the link below-
https://brainly.com/question/32759602
#SPJ11
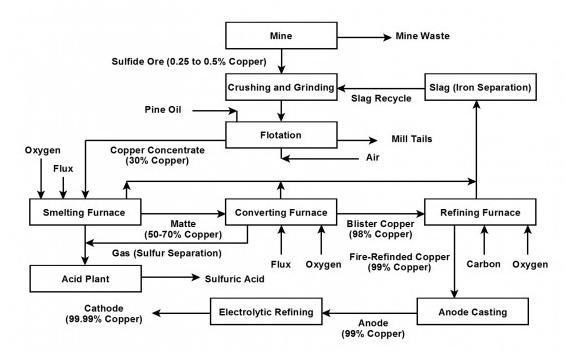
Plz answer these questions :

Answers
Answer: 2) see below
3) 2AgCl → 2Ag + Cl₂
Explanation:
2)
Step 1: Crushing & Grinding CuFeS₂(s)→ CuFeS₂(s)
Step 2: Froth Flotation CuFeS₂(s)----> CuFeS₂ (l)
Step 3: Roasting 2CuFeS₂(l) + 3O₂(g) → 2FeO(s) + 2CuS(s) + 2SO₂(g)
Step 4: Converting matte to blister Cu₂S(l) + O₂(g) → 2Cu(l) + SO₂(g)
Step 5: Anode Casting Cu(l) → Cu(s)
Step 6: Electrolytic Refining Cu(s) → Cu(s)
Anode: Cu(s) → Cu₂ + (aq) + 2e-
Cathode: Cu2 + (aq) + 2e- → Cu(s)
see diagram below for illustration of steps
3) When silver chloride (solid) is exposed to sunlight, it decomposes into silver (solid) and chlorine (gas).
The equation is written as: \(2AgCl_{(s)}\xrightarrow{\text{sunlight}}}2Ag_{(s)}+Cl_{2(g)}\)
* GIVING BRAINLIEST*
3Ca+2 FeCl3 -> 3CaCl2 + 2Fe
Calcium metal + Iron Chloride -> Calcium Chloride + Iron metal
Identify the reason that atoms react with each other.
Answers
i think its double replacement if i'm not mistaken
In 2Fe+3Cl2=2FeCl3, how much iron (Fe) is needed to produce 4 moles of FeCl3?
In 2Fe+3Cl2=2FeCl3, how much FeCl3 is produced when 5 moles of Fe react in excess chlorine?
Answers
Answer:
4 mol Fe
5 mol FeCl₃
Explanation:
Step 1: Write the balanced equation
2 Fe + 3 Cl₂ ⇒ 2 FeCl₃
Step 2: Calculate how much iron (Fe) is needed to produce 4 moles of FeCl₃
According to the balanced equation, the molar ratio of Fe to FeCl₃ is 2:2.
4 mol FeCl₃ × 2 mol Fe/2 mol FeCl₃ = 4 mol Fe
Step 3: Calculate how much FeCl₃ is produced when 5 moles of Fe react in excess chlorine
According to the balanced equation, the molar ratio of Fe to FeCl₃ is 2:2.
5 mol Fe × 2 mol FeCl₃/2 mol Fe = 5 mol FeCl₃
The alloy bronze consists of mostly copper and approximately 12% of what other element?.
Answers
The answer is that bronze consists of mostly copper and approximately 12% tin.
Bronze is an alloy made up of two or more metals, with copper being the primary component. The addition of other metals to copper alters its properties, making it stronger, harder, and more durable.
Tin is the most common metal added to copper to create bronze, comprising approximately 12% of the alloy. Other metals, such as aluminum, nickel, or zinc, may also be added in varying amounts to create specific types of bronze for different purposes.
In summary, the answer to your question is that tin is the other element added to copper to create bronze, with approximately 12% of the alloy consisting of tin.
Bronze is an alloy primarily made up of copper and tin, with tin being the secondary element, usually present in about 12% of the composition. This combination of metals provides the alloy with improved strength, durability, and corrosion resistance compared to pure copper.
To know more about bronze visit:
brainly.com/question/14636013
#SPJ11
Rank the following gases in order of decreasing rate of effusion.
Rank from the highest to lowest effusion rate. To rank items as equivalent, overlap them.
H2
Ar
Ne
C4H8
CO
Answers
The order of decreasing rate of effusion for the given gases is:
H2 > He = Ne > CO > Ar > C4H8
This means that hydrogen (H2) will effuse the fastest, followed by helium (He) and neon (Ne) at the same rate, then carbon monoxide (CO), argon (Ar), and finally butane (C4H8) with the slowest effusion rate. This order is determined by Graham's law of effusion, which states that the rate of effusion of a gas is inversely proportional to the square root of its molar mass. Since hydrogen has the lowest molar mass, it will effuse the fastest, while butane has the highest molar mass and therefore the slowest effusion rate. The other gases fall somewhere in between based on their respective molar masses.
learn more about gases here:
https://brainly.com/question/1369730
#SPJ11
6 ethoxycarbonyl 3 5 diphenyl 2 cyclohexenone molecular weight
Answers
The molecular weight of the 6 ethoxycarbonyl 3 5 diphenyl 2 cyclohexenone is 318.4 g/mol.
The formula for the compound, 6 ethoxycarbonyl 3 5 diphenyl 2 cyclohexenone is :
The molecular formula = C₂₁H₁₈O₃
The molar mass of the C = 12 g/mol
The molar mass of the H = 1.008 g/mol
The molar mass of the O = 16 g/mol
The molecular weight of the compound is :
The molecular weight of C₂₁H₁₈O₃ = ( 21 × 12 + 18 × 1.008 + 3 × 16 )
The molecular weight of C₂₁H₁₈O₃ = 318.4 g/mol.
The molecular weight of C₂₁H₁₈O₃ is 318.4 g/mol.
To learn more about molecular weight here
https://brainly.com/question/20380323
#SPJ4
This question is incomplete, the complete question is :
6 ethoxycarbonyl 3 5 diphenyl 2 cyclohexenone. calculate the molecular weight of the given compound.