Answers
Answer:
option C is correct
Explanation:
Considering the ideal gas law and the definition of Avogadro's Number, the correct option is option a. The temperature of a sample of CH₄ gas (10.34 g) in a 50.0 L vessel at 1.33 atm is 984 °C.
In first place, you have to know that ideal gases are a simplification of real gases that is done to study them more easily.
It is considered to be formed by point particles, do not interact with each other and move randomly. It is also considered that the molecules of an ideal gas, in themselves, do not occupy any volume.
An ideal gas is characterized by three state variables: absolute pressure (P), volume (V), and absolute temperature (T).
The relationship between them constitutes the ideal gas law, an equation that relates the three variables if the amount of substance, number of moles n, remains constant and where R is the molar constant of the gases:
P×V = n×R×T
In this case, being the molar mass of CH₄ being 16 \(\frac{g}{mole}\), that is, the mass present in one mole of an element or compound, the number of moles that 10.34 grams contains is calculated as:
\(10.34 g*\frac{1 mole}{16.04 g} = 0.645 moles\)
So, you know:
P= 1.33 atmV= 50 Ln= 0.645 molesR=0.082 (atm×L)/ (mol×K)T= ?Replacing:
1.33 atm × 50 L= 0.645 moles× 0.082 (atm×L)/ (mol×K) ×T
Solving:
T= [1.33 atm × 50 L] ÷ [0.645 moles× 0.082 (atm×L)/ (mol×K) ]
T≅ 1257 K
Being 273 K equivalent to 0 C, then:
T= 1257 K= 984 C
In summary, the correct option is option a. The temperature of a sample of CH₄ gas (10.34 g) in a 50.0 L vessel at 1.33 atm is 984 °C.
Learn more about the ideal gas law: brainly.com/question/4147359?referrer=searchResults
Related Questions
______ + _______ --> H2O + FrF Complete and balance the equation representing neutralization reaction.
Answers
The general form of a neutralization reaction is HF + FrOH → FrF + H₂O
Which of the following is the formula for a neutralisation reaction?We refer to this as a neutralisation reaction. Only this reaction, which produces NaCl and water as products, is a neutralisation reaction since it involves HCl and NaOH. The resulting response is listed below: NaCl(aq) + H₂O = HCl(aq) + NaOH(aq) (l)
Which of these reactions neutralises an effect?The interaction of H⁺ ions and OH⁻ ions produces water in a neutralisation reaction, which occurs when an acid and a base combine to make water and a salt. The neutralisation of a strong acid and strong base yields a pH of 7.
To know more about reaction visit:-
https://brainly.com/question/28984750
#SPJ1
Which angle is complementary toE0D
Answers
Answer:
?
Explanation:
Is there supposed to be a picture or is that only 5he question?
⚠️LINKS WILL BE REPORTED⚠️ // Need answers as fast as possible!
- Electrons always fill orbitals in the same order. Each s orbital holds 2 electrons, each set of p orbitals holds 6 electrons, each set of d orbitals holds 10 electrons, and each set of f orbitals holds 14 electrons. The order in which orbitals are filled, from first to last, is:
1s 2s 2p 3s 3p 4s 3d 4p 5s 4d 5p 6s 4f 5d 6p 7s 5f 6d 7p
Beryllium has 4 electrons. What is the electron configuration of beryllium?
A.
2s^22p^2
B.
1s^22s^2
C.
2s^4
D.
4p^1
Answers
Answer:
\(1s^2\, 2s^2\).
Explanation:
Electron orbitals in an atom (e.g., \(1s\)) are denoted with:
A number, denoting the shell (principal energy level) of this orbital, andA letter, denoting the shape of this orbital (\(s\), \(p\), \(d\), etc.)
There are two aspects to consider when finding the electron configuration of an atom:
The number of electrons that each type of orbitals could hold, andThe order in which the orbitals are filled.The \(s\) orbital in each shell could hold up to \(2 \times 1 = 2\) electrons (one \(s\!\) orbital per shell, with up to two electrons.)
The \(p\) orbitals in each shell could hold up to \(2 \times 3 = 6\) electrons (three \(p\!\) orbitals per shell, with up to two electrons in each orbital.)
The \(d\) orbitals in each main shell could hold up to \(2 \times 5 = 10\) electrons (five \(d\!\) orbitals per shell, with up to two electrons in each orbital.)
Refer to the order in which the orbitals are filled (Aufbau principle.)
The first orbital to be filled would be \(1s\) (the \(s\) orbital of the first shell,) accommodating up to \(2\) electrons.The second orbital to be filled would be \(2s\) (the \(s\) orbital of the second shell,) accommodating up to \(2\) electrons.All four electrons of Beryllium are thus assigned to the \(1s\) and \(2s\) orbitals. In a ground-state Beryllium atom, orbitals \(2p\) and beyond would contain no electrons.
Notation:
Two electrons in the \(1s\) orbital: \(1s^{2}\) (the superscript denotes the number of electrons in this orbital (or group of orbitals).)Two electrons in the \(2s\) orbital: \(2s^2\).Write the non-empty orbitals in the order by which they are filled:
\(1s^2\, 2s^2\).
Plants use sunlight as energy to convert carbon dioxide and water into glucose and oxygen. Which best describes
the reaction?
O The law of conservation of mass indicates the same amount of carbon will be found in the reactants as in the
products.
O The law of conservation of energy indicates the same amount of carbon will be found in the reactants as in the
products.
O The law of conservation of mass indicates the same amount of glucose will be found in the reactants as in the
products
O The law of conservation of energy indicates the same amount of glucose will be found in the reactants as in the
products
Answers
Answer:
The law of conservation of mass indicates the same amount of carbon will be found in the reactants as in the products.
Explanation:
Question:
Plants use sunlight as energy to convert carbon dioxide and water into glucose and oxygen. Which best describes the reaction?
Options:
The law of conservation of mass indicates the same amount of carbon will be found in the reactants as in the products. The law of conservation of energy indicates the same amount of carbon will be found in the reactants as in the products. The law of conservation of mass indicates the same amount of glucose will be found in the reactants as in the products. The law of conservation of energy indicates the same amount of glucose will be found in the reactants as in the products.Answer:
A.) The law of conservation of mass indicates the same amount of carbon will be found in the reactants as in the products.
Explanation:
I just did the test on edge 2020 and got it right!
Predict the missing component in the nuclear equation.

Answers
Since what we have is an alpha decay, the missing component is 234/90 Th
What is Alpha decay?Alpha decay is a type of radioactive decay in which an atomic nucleus emits an alpha particle, which is a helium nucleus consisting of two protons and two neutrons. During alpha decay, the atomic number of the parent nucleus decreases by two, and the mass number decreases by four.
Apha particles can be stopped by a thin layer of material such as paper or skin, and they do not penetrate very far into matter.
Learn more about Alpha decay:https://brainly.com/question/27870937
#SPJ1
1. What is the density, in g/cm², of a substance weighing 22.2 cg with dimensions of 0.333
cm by 0.444 cm by 0.555 cm?
Answers
Volume:-
\(\\ \tt\longmapsto 0.333(0.444)(0.555)=0.08cm^3\)
So
\(\\ \tt\longmapsto Density=\dfrac{Mass}{Volume}\)
\(\\ \tt\longmapsto Density=\dfrac{2.22}{0.08}=27.75g/cm^3\)
How many molecules are in 6.5 moles of hydrochloric acid, HCl?
Answers
Answer:
There are two moles HCl formed for each mole of Cl2, hence 2 x 0.141 = 0.282 moles HCl is produced. = 0.282 x (1.008 + 35.45) = 10.3 g. (1.)
which of the following are examples of single replacement reactions select all that apply.
1) Ca(OH)2(aq) + 2 HCl(aq) ----> CaCl2(aq) + 2 H2O(l)
2)Mg(s) + Zn(NO3)2(aq) ------> Mg(NO3)2(aq) + Zn(s) 3)Na2S(aq) + Cd(NO3)2(aq) ----> 2 NaNO3(aq)
4) K(s) + 2HCl(aq) ----> 2KCl (aq) +H2(g)
Answers
Answer:
4
Explanation:
please mark brainiest
K(s) + 2HCl(aq) ----> 2KCl (aq) +H2(g) is an example of single replacement reactions.
What do you mean single replacement reactions?A single-displacement reaction, also known as single replacement reaction or exchange reaction, is a chemical reaction in which one element is replaced by another in a compound.
Single-replacement reactions always involve two pure elements and one aqueous compound/solution. In the above reaction, the A and C would be pure elements.
A single replacement reaction, sometimes called a single displacement reaction, is a reaction in which one element is substituted for another element in a compound.
Learn more about single replacement reactions:
https://brainly.com/question/13903783
#SPJ2
I observed evidence technician Josh Sanders dust the inside doorknob on the front door for latent fingerprints. Dusting the doorknob did reveal latent prints, which Sanders lifted with tape and transferred to a small card. That occurred at approximately 3:15 p.m. I later observed Detective Laura Wilson collect a bullet casing from the tile floor in the kitchen after another investigator first photographed the area. The bullet casing was found on the floor beneath the kitchen table and was picked up by Wilson (with gloved hands) and placed in an evidence bag. Collection of the bullet casing occurred at 3:42 p.m. I then looked in the guest bathroom down the hall from the kitchen and observed another evidence technician, William Soto, collecting unknown red fibers from inside the bathroom sink. The time was approximately 3:53 p.m. Soto collected the red fibers with tweezers and placed them inside an evidence bag.
Answers
The chain of custody for the finger print on the Door Know is summarized in the Chain of Custody form attached.
What is a chain of custody?This is a procedure that documents each individual who handled the evidence, the date/time it was gathered or transferred, and the purpose for the transfer as it moves through its collecting, safekeeping, and analysis lifespan.
The most important aspect of evidence recording is the chain of custody. It is essential to demonstrate to the court of law that the evidence is genuine, i.e., that it was obtained at the crime scene. It was always in the care of someone assigned to manage it, and it was never unaccounted for.
The chain of custody demands that every transfer of evidence from person to person be documented from the time the evidence is acquired and that it be proven that no one else could have accessed that evidence. It is recommended to restrict the number of transfers to a minimum.
Learn more about the chain of custody:
https://brainly.com/question/17899853?
#SPJ1
Full Question:
In the video at the beginning of this unit, you were introduced to Jordan, a college student and newspaper intern. Jordan was shadowing police who were collecting evidence at the scene of a crime.
Jordan is shadowing the police again at a different crime scene. This time you have gone with him. Your job will be to record all of the required information on a chain of custody document.
Choose one of the items of physical evidence mentioned in Jordan's notes below.
Complete the chain of custody document for that item. (Make it similar to the example provided beneath Jordan's notes.)
Jordan's Notes
Tuesday, February 5
I observed evidence technician Josh Sanders dust the inside doorknob on the front door for latent fingerprints. Dusting the doorknob did reveal latent prints, which Sanders lifted with tape and transferred to a small card. That occurred at approximately 3:15 p.m.
I later observed Detective Laura Wilson collect a bullet casing from the tile floor in the kitchen after another investigator first photographed the area. The bullet casing was found on the floor beneath the kitchen table and was picked up by Wilson (with gloved hands) and placed in an evidence bag. Collection of the bullet casing occurred at 3:42 p.m.
I then looked in the guest bathroom down the hall from the kitchen and observed another evidence technician, William Soto, collecting unknown red fibers from inside the bathroom sink. The time was approximately 3:53 p.m. Soto collected the red fibers with tweezers and placed them inside an evidence bag.
Example of a Chain of Custody Form
Chain of CustodyItem collected as evidence: Individual who collected the item: Date and time of collection: Description of location where item was collected: Method of collection:
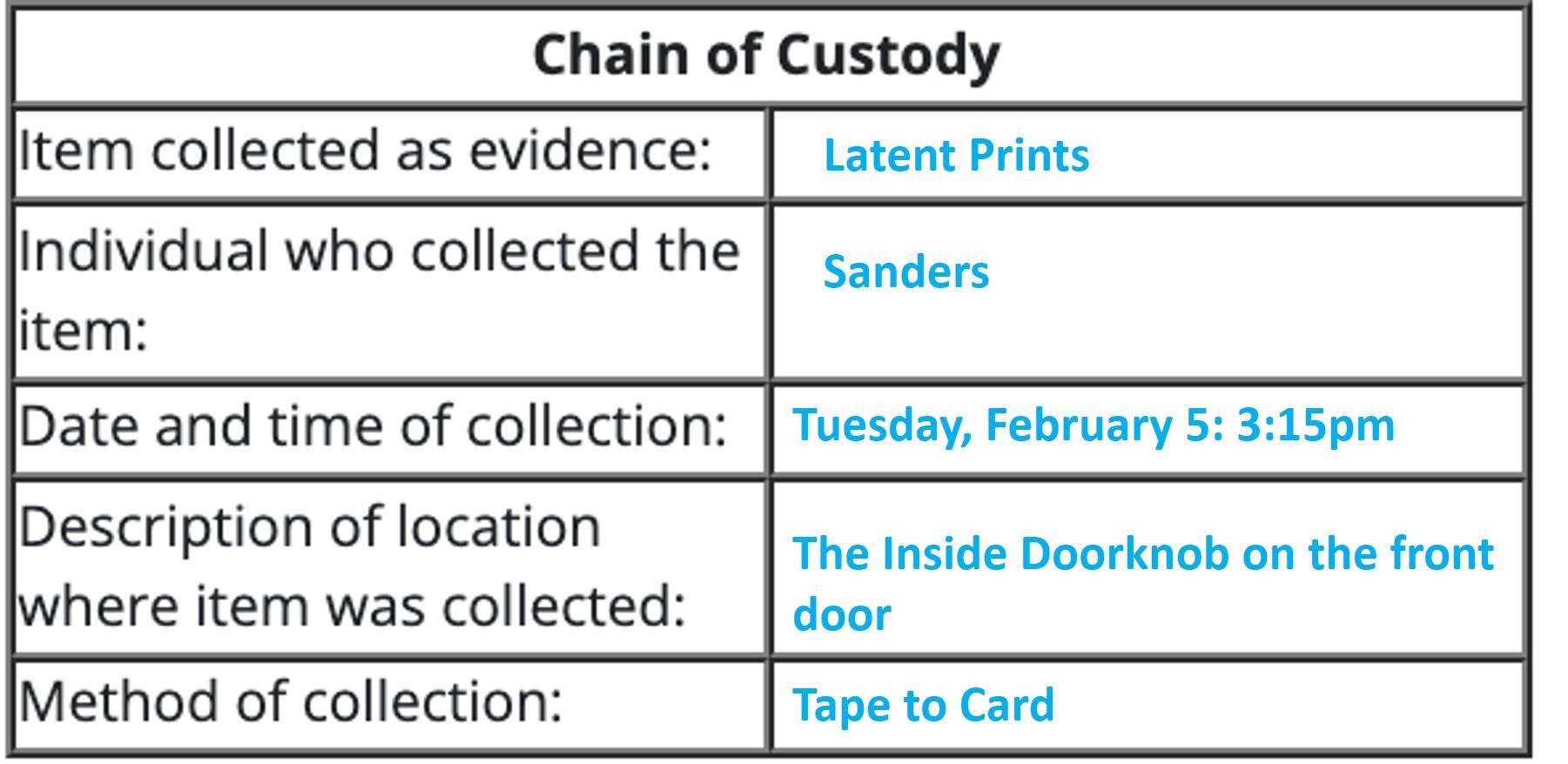
plate movements on earths crust creates?
Answers
Answer:
Earthquakes and Volcanos?
Explanation:
I'm a little unsure about my answer, however, I do have some insight backing my answer. Earthquakes happen when the plate tectonics shift into each other, causing them to collide. Then for volcanos, the plate tectonics shift from convection current and molten rock from the mantle come to the surface.
Are ALL acids and based
harmful to humans?
Answers
a metal worker used a cutting torch that operated by reacting acetylene gas with oxygen gas, as shown in the unbalanced equation below. balance the following equation for the reaction of acetylene and oxygen, using the smallest whole-number coefficients. (the values are 1,2,3,4,5)

Answers
The balanced equation for the reaction of acetylene (C₂H₂) and oxygen (O₂) is; 2 C₂H₂(g) + 5 O₂(g) → 4 CO₂(g) + 2 H₂O(g) + heat
The coefficients in the balanced equation represent the stoichiometric ratio of the reactants and products in the chemical reaction. In this case, 2 molecules of acetylene (C₂H₂) react with 5 molecules of oxygen (O₂) to produce 4 molecules of carbon dioxide (CO₂) and 2 molecules of water (H₂O), along with the release of heat.
The balanced equation shows that the number of atoms of each element is the same on both the sides of the equation, in accordance with the law of conservation of mass.
To know more about acetylene here
https://brainly.com/question/20529866
#SPJ1
6. What are floral preservatives?
Answers
Floral preservatives are chemical solutions used to extend the longevity of cut flowers after they have been harvested.
These solutions contain a mixture of substances designed to optimize the growth and development of flowers while inhibiting the growth and activity of harmful bacteria, fungi, and other microorganisms.
Typically, floral preservatives contain three primary components: a source of carbohydrates (like sugar) to feed the flower, an acidifying agent (like citric acid) to lower the pH and prevent growth of bacteria, and a biocide (like bleach or silver nitrate) to prevent the growth of microbial contaminants.
The carbohydrates in the preservative solution provide energy and nutrients to the flowers, allowing them to continue to develop and open up. The acidifying agent helps to lower the pH of the solution, preventing the growth of bacteria that can cause the blockage of stem tissues in the vessel. The biocide inhibits microbial growth and prevents the growth and spread of disease-causing organisms that may be present.
Floral preservatives are effective in increasing the length of time that cut flowers can remain fresh and attractive, providing benefits to florists, retailers, and consumers alike.
For such more questions on preservative
https://brainly.com/question/15777386
#SPJ11
please help asap will give brainiest What is the independent variable in an experiment? A) The variable that remains the same throughout the experiment. B) The variable being tested in the experiment. C) The variable being measured in an experiment.
Answers
PLEASE ANSWER QUICKLY!!!!
2KI (aq) + Cl₂(g) → 2KCl(aq) + 1₂(g)
What volume of 12 gas forms when
21 L Cl2 react at STP?
[?] L 12

Answers
The volume of 12 gas forms when 21 L Cl2 react at STP is 21 L.
To determine the volume of 12 gas (I assume you mean I2 gas) formed when 21 L of Cl2 reacts at STP (standard temperature and pressure), we need to use the ideal gas law equation.
The ideal gas law equation is given by:
PV = nRT
Where:
P = pressure
V = volume
n = number of moles
R = ideal gas constant
T = temperature
At STP, the pressure is 1 atm, and the temperature is 273.15 K.
From the balanced equation, we can see that the molar ratio between Cl2 and I2 is 1:1. So, if 21 L of Cl2 reacts, it will produce an equal volume of I2 gas.
Given that the volume of Cl2 is 21 L, we can assume the volume of I2 gas formed will also be 21 L.
Therefore, the volume of I2 gas formed is 21 L.
For more such questions on volume
https://brainly.com/question/1749900
#SPJ8
Which solids are insoluble in water.
Answers
Some types of solids that are insoluble in water are:
Metals. (most of them)Non-Metallic ElementsMetal OxidesSome Non-Metallic ElementsMetal Carbonates (most of them)Metal Sulfides (most of them)Salts (some of them)Which solids are insoluble in water?Many solids are insoluble in water, meaning they do not dissolve in water to a significant extent. Here are some examples of common solids that are generally insoluble in water:
Metals: Most metals, such as gold, silver, platinum, and copper, are insoluble in water.
Non-Metallic Elements: Many non-metallic elements, such as carbon (in the form of graphite or diamond), sulfur, phosphorus, and iodine, are insoluble in water.
Metal Oxides: Some metal oxides, particularly those of less reactive metals, are insoluble in water. Examples include aluminum oxide (Al2O3), iron(III) oxide (Fe2O3), and lead(II) oxide (PbO).
Metal Carbonates: Most metal carbonates are insoluble in water. Examples include calcium carbonate (CaCO3), lead(II) carbonate (PbCO3), and copper(II) carbonate (CuCO3).
Metal Sulfides: Many metal sulfides are insoluble in water. Examples include lead(II) sulfide (PbS), silver sulfide (Ag2S), and mercury(II) sulfide (HgS).
Insoluble Salts: Certain salts have limited solubility in water. Examples include silver chloride (AgCl), lead(II) iodide (PbI2), and calcium sulfate (CaSO4).
It's important to note that while these solids are generally insoluble in water, they may exhibit some solubility to a small extent. The solubility of a solid in water can vary depending on factors such as temperature, pressure, and the presence of other solutes.
Learn more about solubility:
https://brainly.com/question/23946616
#SPJ1
For a concave mirror, incident light beams through C will reflect:
(a) back through C
(b) through F
(c) parallel to PA
Answers
Answer:
c
Explanation:
Carla is using a fertilizer that contains nitric acid. How is nitric acid classified?
strong acid
weak acid
strong base
weak base
Answers
Answer:
Strong acid.....(A)
Explanation:
hope this helps, the person above me is also correct
Answer:
a
Explanation:
6. How many moles are in 8.30 x 1023 molecules of CO₂?
a.
b.
C.
d.
1.37
2.8
55.5
100
Answers
Select all of the following that are combustion reactions.

Answers
Answer:
Explanation:
1,2,4,
The equations that show combustion are equations A, B and D.
What is combustion?When we talk about combustion, the idea is that the substance would be burnt in oxygen. In other words, the combustion can be taken to be an oxidation reaction. It is an oxidation reaction in the sense that the oxidation number of the substance that is reacting with the oxygen would become increased.
When we look at the equations that we have, it is quite easy to pick out among the balanced reaction equations that are shown here the ones that has to do with the burning of the substance in oxygen and a consequent rise in the oxidation number.
Learn more about combustion:https://brainly.com/question/15117038
#SPJ1
there are several elements whose atoms make more than one type of ion. Where in
the periodic table are these elements usually found?
Answers
Answer:
Explanation:
These are the transition metals. Groups 3-12 also know as the "d" block elements
Convert the temperature of scalding water 54 into degrees Fahrenheit and kelvin
Answers
The temperature of the water in degrees Fahrenheit and kelvin are:
129.2 °F327.15 KHow to convert the temperature ?To convert the temperature of scalding water, 54 degrees Celsius, into degrees Fahrenheit, you can use the following formula:
F = (°C x 9/5) + 32
So:
(54 x 9/5) + 32 = 129.2 °F
To convert the temperature of scalding water, 54 degrees Celsius, into Kelvin, you can use the following formula:
°C + 273.15 = K
So,
54 + 273.15 = 327.15 K
Find out more on temperature at https://brainly.com/question/3994464
#SPJ1
Explain how these results show that chlorine is more reactive than bromine and
lodine.
Answers
Chlorine is more reactive than bromine because it replaces both bromine and iodine.
How chlorine is more reactive than bromine?Fluorine is the most sensitive while on the other hand, the astatine is the least reactive as compared to other elements. The chlorine displaces both bromine and iodine, and bromine displaces iodine because of its high reactivity. The element that replaces other atom is considered as more reactive.
The order of reactivity is that the chlorine is more reactive than bromine, which indicates that chlorine is more reactive than iodine.
So we can conclude that chlorine is more reactive than bromine due to high reactivity.
Learn more about Chlorine here: https://brainly.com/question/24218286
#SPJ1
A balloon is filled to a volume of 2.20L at a temperature of 25.0*C. The balloon is then heated to a temperature of 51*C. Find the new volume of the balloon
Answers
The new volume of the balloon after heating it to a temperature of 51 °C is approximately 2.39 L.
What is the final volume of the balloon?Charles's law states that "the volume occupied by a definite quantity of gas is directly proportional to its absolute temperature.
It is expressed as;
\(\frac{V_1}{T_1} =\frac{V_2}{T_2}\)
Given that:
Initial temperature of gas T₁ = 25°C = (25.0 + 273.15) = KInitial volume of gas V₁ = 2.2 LFinal temperature T₂ = 51 °C = ( 51 + 273.15 ) = 324.15 KFinal volume V₂ = ?Substituting the given values and solve for V₂:
\(V_1T_2 = V_2T_1\\\\V_2 = \frac{V_1T_2}{T_1} \\\\V_2 = \frac{2.2\ *\ 324.15}{298.15 }\\ \\V_2 = 2.39 \ L\)
Therefore, the final volume is 2.39 litres.
Learn more about Charles's law here: https://brainly.com/question/23122443
#SPJ1
Like Father Like Mother What is the best summary of this oassage
Answers
Answer:
hope this helps <3
Explanation:
Landon glances across the dinner table and thinks to himself that his fathers curly hair looks just like his own. He wishes his hair were straight like his mothers because he thinks he looks better with straight hair. Landon works hard to keep it straighter by combing and using hair gel, but it usually curls right back up. He has his mothers nose, but not her hair, unlike his sister Emily, who is also the result of the combination of the DNA from both of his parents and has straight hair like their mothers. Most eukaryotic organisms, like Landon, are formed through the process of sexual reproduction. Landon realizes that he and Emily are evidence that sexual reproduction, the combination of their two parents, creates diverse offspring.
Landons dinner plate is filled with one of his favorite foods-mashed potatoes made from potatoes fresh from his grandmothers garden. He remembers helping her get ready to plant the potatoes in early spring. They cut each potato into several pieces and planted the pieces in a nice straight row in the garden. When these eukaryotic plants came up, he noticed that each plant looked like all the others. These identical offspring with uniform traits are the result of asexual reproduction. Landons grandmother grew potatoes by cutting the whole potato into smaller pieces, which is a type of asexual reproduction called vegetative propagation.
After dinner, Landon wanted to go watch television, but his mother insisted that he put the leftover food in the refrigerator. Perhaps she knew that the cool temperature inside the refrigerator would stop the growth of bacteria, which are prokaryotic and can reproduce asexually in as little as 20 minutes. This rapid asexual reproduction, called binary fission, would produce thousands and thousands of identical bacterial cells that could cause Landons tasty leftover food to spoil before the end of his favorite show.
Landon finishes up the dishes and puts away the leftovers. As he sets the last dinner glass back into the cupboard, he catches a glimpse of his curly hair in his reflection. He thinks to himself that his mothers nose and his fathers hair are actually a good combination that looks pretty good on him
Freezing point depression is a colligative property.The freezing point of pure water is 0.0°C. How many grams of ethylene glycol (C2H6O2) must be mixed in 100.0 g of water to lower the freezing point of the solution to -4.3°C?_______ g
Answers
Explanation:
The freezing point depression is a colligative property. We have to find the mass of ethylene glycol that we have to add to 100.0 g of water to change its freezing point from 0.0 °C to -4.3 °C.
The freezing point depression for a solution can be calculated using the following equation:
ΔTf = kf * molality * i
Where ΔTf is the freezing point depression, kf is the freezing point depression constant (it depends on the solvent and for water is 1.86 °C/m), molality is the molality of the solution and i is the Van't Hoff factor.
The Van't Hoff factor represents the number of particles formed when that compound dissolves. In our case the solute is ethylene glycol, a covalent compound, so it won't form ions when dissolved in the water. Then i is equal to 1.
The temperature must change from 0.0 °C to -4.3 °C, then the freezing point depression is 4.3 °C. So we know that:
i = 1 kf = 1.86 °C/m ΔTf = 4.3 °C
We can replace those values in the formula and find the molality of the solution.
ΔTf = kf * molality * i
4.3 °C = 1.86 °C/m * molality * 1
molality = 4.3 °C/(1.86 °C/m)
molality = 2.31 m
Now we can get the moles of ethylene glycol from the definition of the molal concentration. Molality are the moles of solute per kg of solvent. The mass of water is 100 g.
mass of solvent = 100.0 g * 1 kg/(1000 g)
mass of solvent = 0.100 kg
molality = moles of solute/(mass of solvent in kg)
moles of solute = molality * mass of solvent in kg
moles of solute = 2.31 m * 0.100 kg
moles of solute = 0.231 moles
And finally we can convert the moles of ethylene glycol to grams using its molar mass.
molar mass of C₂H₆O₂ = 62.07 g/mol
mass of C₂H₆O₂ = 0.231 moles * 62.07 g/mol
mass of C₂H₆O₂ = 14.4 g
Answer: 14.4 g of ethylene glycol must be mixed.
The picture shows two cars moving at the same speed. Which car has more kinetic energy?
Answers
Answer:
car 2/red
Explanation:
give me crown
What type of cells are gametes?
Answers
Answer:
Reproductive cells(also known as sex cells) are gametes.
Explanation:
Have a great day :)
Answer:
Gametes are an organism's reproductive cells
Explanation:
Choose the option that would convert mg/L
into molarity (mol/L).
A. Convert mg/L to get g/L then multiply by the volume (L).
B Convert mg/L to g/L then divide by molar
mass (g/mol).
C. Convert mg/L to g/L then divide by the volume (L).
D. Convert mg/L to g/L then divide by the
number of moles (mol).
Answers
The correct option that would convert mg/L into molarity (mol/L)- Convert mg/L to get g/L then multiply by the volume (L). so, option (a) is correct.
What is molarity ?
The amount of solute in one mole per liter of solution is known as molarity. For instance, when table salt is dissolved in water, the solute is salt, and the solution is water. 58.44 grams make up one mole of sodium chloride. One molar solution, often known as 1M, is created when 58.44 grams of sodium chloride are dissolved in one liter of water.
What is volume ?
Volume, a three-dimensional quantity, is used to calculate the capacity of a solid shape. It suggests that the volume of a closed figure determines the amount of three-dimensional space it can occupy.
Therefore, the correct option that would convert mg/L into molarity (mol/L)- Convert mg/L to get g/L then multiply by the volume (L). so, option (a) is correct.
Learn more about molarity from the given link.
https://brainly.com/question/26873446
#SPJ1
Answer: Not A, its B
Explanation: B
Which of the following behaviors might indicate a patient is drug seeking?
A. A patient wants to avoid a specific medication because of
potential side effects.
B. A patient explains that she is from out of town and needs a
specific medicine because she left her prescription at home.
C. A patient fears her new prescription will conflict with another
medication she's currently taking.
D. A patient thinks she needs a smaller dose of her prescription
because it gives her headaches.
SUBMIT
Answers
Answer:
B is correct :)
Explanation:
Trust me I just took the test