The table shows properties of the most abundant isotope of each of four silver-colored metals.
Element
Number of
Protons
Number of
Neutrons
Platinum (Pt)
78
117
Silver (Ag)
47
60
Nickel (Ni)
28
30
Tin (Sn)
50
70
Which statement best describes a trend shown in the table?
You may use the calculator.
As the number of neutrons increases, the number of electrons decreases.
As the number of protons increases, the number of neutrons decreases.
The number of neutrons is equal to the number of protons, multiplied by a constant.
Each isotope has an equal number of protons and electrons.
Number of
Electrons
78
47
28
50

Answers
Answer: Each isotope has an equal number of protons and electrons.
Related Questions
I need help with part b of this question

Answers
0.6844
Don't ask how I got the answer, just look up Omnicalculator grams to moles or maybe keep a diagram handy like this one

Question 4
Write a chemical reaction depicting what happens to the sugar in water.
Answers
Answer:
When sugar dissolves,these whole sucrose molecules separate from one another
Which of the images shows a phase of matter where there is no specific shape or volume?

Answers
Which of the following does NOT describe elements? can join together to form compounds all the particles are alike can be broken down into simpler substances have unique sets of properties
Answers
Answer:
Can be broken down into simpler substances.
Explanation:
Elements are made of atoms, and that is the simplest understood unit of the universe. :) nOw GiB mY 50000 points.
Answer:
Can be broken down into simpler substances
Explanation:
Because atoms cannot be created or destroyed in a chemical reaction
The balance in the lab is broken! You need to find the mass of a sample of silver with a density of 10.49 g/mL. When the piece of silver is placed into 25.00 mL of water, the volume reads 28.54 mL. What is the mass of this piece of silver?
(A) 262.3 g
(B) 37.1 g
(C) 299.4 g
(D) 0.337 g
(E) 2.721 g

Answers
Answer:
B 37.1 g
Explanation:
find the volume of the displaced water 28.54mL - 25.0mL = 3.54 mL displaced by the silver.
3.54 mL x 10.49 g/mL = 37.1g of total silver
The graph represents the amount of energy in the
reactants and products of a chemical reaction.
If total energy is conserved, which statement
explains why the energy of the reactants does not
equal the energy of the products?
Energy
Energy of Energy of
Reactants Products
A.
The reaction is exothermic
B. The reaction is endothermic.
C. The reaction requires a catalyst.
D
The reaction has a low activation energy.
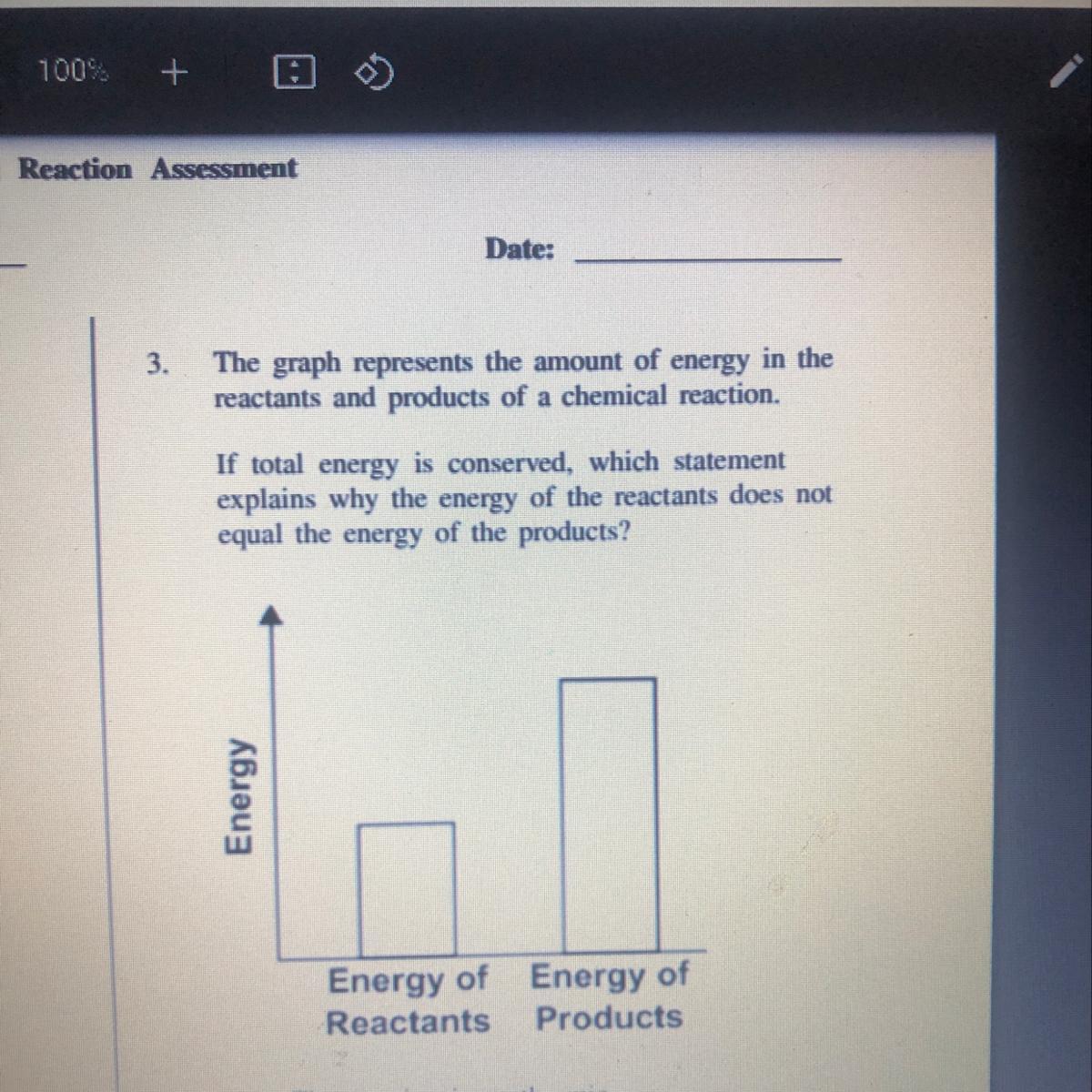
Answers
Answer: The correct answer is in an exothermic reaction the energy of the product is less than the energy of the reactants.
Explanation: got it
The energy of reactants is not equal to that of products as the reaction is an exothermic reaction.
What is an exothermic reaction?
An exothermic reaction is defined as a chemical reaction which involves release of energy in the form of light,heat .In these reactions, energy is transferred from system to surroundings rather than taking energy from surroundings into system as in endothermic reactions.
In an exothermic reaction,change in enthalpy is negative.Therefore, it can be inferred that net amount of energy which is required to start the exothermic reaction is less than the net amount which is released by the reaction.
Examples of exothermic reactions are combustion reactions, detonation of nitroglycerin , neutralization reactions and nuclear fission.
Learn more about exothermic reactions,here:
https://brainly.com/question/28909381
#SPJ2
How does the heat flow from the coffee to the part of the spoon in the coffee?
Answers
Answer:
Heat flows from the coffee to the spoon through conduction
Explanation:I did the lab assignment
Answer:
Heat can travel from one place to another in three ways: Conduction, Convection and Radiation. As the spoon is in direct contact with hot mug of coffee the heat will transfer to spoon this represent the process of conduction.
explain how essential nutrients (water, carbon and nitrogen) are cycled throughout an ecosystem. then identify how that process is different from how energy flows throughout an ecosystem.
Answers
Essential nutrients such as water, carbon and nitrogen are cycled throughout an ecosystem in a process known as biogeochemical cycling.
What is ecosystem?Ecosystem is a community of living organisms and their physical environment. It is a complex network of interactions between living and nonliving elements. These elements include air, sunlight, soil, water, plants, animals and microorganisms. Ecosystems are constantly changing and adapting to their environment, and the interactions between their components create a dynamic balance. Healthy ecosystems provide a variety of benefits, including clean air, clean water, and food. They also provide habitats for many species and are an important part of our global environment.
This process involves the movement of these chemicals through different parts of the environment, such as the atmosphere, land, and water, as well as between living organisms and the environment.
The cycling of essential nutrients begins with the breakdown of organic compounds, such as dead plant and animal matter, by bacteria, fungi, and other organisms. This process releases the nutrients back into the environment, where they can be taken up by other organisms or re-enter the atmosphere. The water cycle is the most basic example of this process, as water evaporates from the land and is returned to the ground as precipitation. Carbon is cycled through the atmosphere, land, and oceans in a process known as the carbon cycle, while nitrogen is cycled through the atmosphere, soil, and water in the nitrogen cycle.
To know more about nitrogen click-
https://brainly.com/question/1380063
#SPJ4
An electron has a
charge.
Answers
An electron has a negative charge.
The charge of an electron is a fundamental property of the particle, and it is denoted by the symbol "e." The magnitude of the charge of an electron is approximately 1.602 × 10^-19 coulombs (C). This value is considered the elementary charge and is used as a reference for other charges. The charge of an electron plays a significant role in determining the behavior and interactions of atoms and molecules. It is opposite in sign to the charge of a proton, which is positive. The electron's charge allows it to interact with other charged particles, such as protons and ions, through electrostatic forces. Electrons are subatomic particles that orbit the nucleus of an atom in specific energy levels or orbitals. They contribute to the overall stability and chemical properties of atoms and participate in chemical bonding and reactions. The movement of electrons between atoms is what enables the formation of chemical bonds and the sharing or transfer of electrons to create ions. In summary, the charge of an electron is negative, and it plays a fundamental role in the structure and behavior of atoms and molecules.
for more questions on electron
https://brainly.com/question/26084288
#SPJ8
A scientist encounters an unknown culture of cells in a laboratory. The only tools available to the scientist to determine the type of cell (animal, prokaryotic, fungal, plant, etc.) are enzymes that degrade particular elements of the extracellular matrix of cells of particular types. Which of the following data would indicate that the unknown cells were from an animal source?
a. The cells are disrupted by application of a lipase (an enzyme that degrades lipids).
b. The cells are disrupted by application of collagenase (an enzyme that degrades collagen).
c. The cells are disrupted by application of cellulase (an enzyme that degrades cellulose).
d. The cells are disrupted by application of lysozyme (an enzyme that degrades peptidoglycan).
Answers
The data that would indicate that the unknown cells are from an animal source is the correct option is b. The cells are disrupted by application of collagenase (an enzyme that degrades collagen).
The data that the cells are the disrupted by the application of collagenase that is an enzyme that degrades collagen. The Animal cells are the standard of the eukaryotic cell, that is enclosed through a plasma membrane. It contains the membrane in certain nucleus and organelles.
The animal cell is a type of the eukaryotic cell that is in lacks of a cell wall and has a true, membrane that is bound to nucleus along with the other cellular organelles.
To learn more about cells here
https://brainly.com/question/18914457
#SPJ4
explain order of reaction and use the data below and the rate equation to show how it is calculated.
Using the data above determine
(a) order with respect to (A)
(b) order with respect to (B)
(c) rate equation
(d) overall order

Answers
The rate equation is Rate = k[A]²[B]³, and the reaction has an overall order of 5.
If the rate equation is correct, what is the reaction's order?A rate law illustrates how a chemical reaction's rate is influenced by the reactant's concentration. The rate law typically has the formula rate = k[A]n for reactions like aA products, where k is the proportionality constant also known as the rate constant. and The reaction's sequence in relation to A is indicated by n.
Rate = k[A]x[B]y
Rate = k[A]²[B]³
Overall order = 2 + 3 = 5
Therefore, the overall order of the reaction is 5, and the rate equation is Rate = k[A]²[B]³.
To know more about reaction visit:-
https://brainly.com/question/28984750
#SPJ1
What forms of energy are produced when
fossil fuels burn?
Answers
When fossil fuels burn, several forms of energy are produced, including:
Heat energy: The primary form of energy released during fossil fuel combustion is heat. Fossil fuels contain chemical energy stored for millions of years, and when they burn, this energy is released in the form of heat. The heat energy can be harnessed for various purposes, such as heating buildings or generating steam to drive turbines.
Light energy: Burning fossil fuels can also produce light energy in the form of flames or glowing embers. This light energy is a byproduct of combustion.
Mechanical energy: Heat generated by burning fossil fuels can be converted into mechanical energy. This is typically achieved by using heat to produce steam, which drives a turbine connected to a generator. The rotating turbine converts the heat energy into mechanical energy, which is further transformed into electrical energy.
Electrical energy: Through the process described above, burning fossil fuels can ultimately generate electrical energy. The mechanical energy produced by the turbine is converted into electrical energy by the generator. Electrical energy can power various devices, appliances, industries, and infrastructure.
It's critical to note that while burning fossil fuels can produce useful forms of energy, it also results in the release of carbon dioxide and other greenhouse gases. This contributes to climate change and environmental concerns. As a result, there is a global shift towards cleaner and renewable energy sources to mitigate these negative impacts.
What mass of sodium hydroxide, NaOH, would be required to produce 16 g of the antacid milk of magnesia [magnesium hydroxide, Mg(OH)2] by the following reaction? MgCl2(aq) + 2NaOH(aq) ⟶ Mg(OH)2(s) + 2NaCl(aq)
Answers
It would take 22g of sodium hydroxide (NaOH) to make 16g of the antacid milk of magnesia (magnesium hydroxide).
Simply put, what is stoichiometry?In the field of chemistry known as stoichiometry, desired quantitative data is ascertained by using relationships between the reactants and/or products of a chemical reaction. Stoichiometry literally translates as the measure of elements because the Greek words stoikhein and metron both mean element and measure, respectively.
What is the stoichiometric law?In a chemical reaction, the total mass of reactant and product are equal, according to the statement, and neither is generated nor destroyed. This is the stoichiometric law, and also the law of conservation of mass.
\(16 \mathrm{~g} \text { of } \mathrm{Mg}(\mathrm{OH})_2 \times \frac{1 \mathrm{molMg}(\mathrm{OH})_2}{58.3 g \mathrm{gg}(\mathrm{OH})_2} \times \frac{2 \mathrm{~mol} \mathrm{NaOH}}{1 \mathrm{molMg}(\mathrm{OH})_2} \times \frac{40 \mathrm{gNaOH}}{\mathrm{molNaOH}}=22 \mathrm{~g}\)
To learn more about Stoichiometry visit:
brainly.com/question/29775083
#SPJ1
Percent Change of pH = 100% x ( pH at 5 drops - pH at 0 drops ) / ( pH at 0 drops )
Answers
The formula you provided calculates the percent change in pH based on the difference between the pH at 5 drops and the pH at 0 drops. Here's how you can use the formula:
1. Determine the pH at 5 drops and the pH at 0 drops.
Let's say the pH at 5 drops is 4 and the pH at 0 drops is 7.
2. Plug the values into the formula:
Percent Change of pH = 100% × (pH at 5 drops - pH at 0 drops) / pH at 0 drops
Percent Change of pH = 100% × (4 - 7) / 7
3. Calculate the numerator:
4 - 7 = -3
4. Calculate the denominator:
Percent Change of pH = 100% × (-3) / 7
5. Calculate the percent change:
Percent Change of pH = -300% / 7
Therefore, the percent change in pH from 0 drops to 5 drops is approximately -42.86%.
What is the final temperature after 840 Joules is absorbed by 10.0g of water at 25.0
C?
Answers
The final temperature of the water is: T_final = 45.0°C
We can use the formula for the specific heat capacity of the water to solve this problem:
q = mcΔT
First, we can calculate the initial energy of the water:
q = mcΔT
q = (10.0 g) (4.184 J/g°C) (25.0°C)
q = 1,046 J
Next, we can calculate the final temperature after absorbing 840 J:
q = mcΔT
840 J = (10.0 g) (4.184 J/g°C) (ΔT)
ΔT = 20.0°C
Therefore, the final temperature of the water is:
T_final = T_initial + ΔT
T_final = 25.0°C + 20.0°C
T_final = 45.0°C
To know more about final temperature, here
brainly.com/question/11244611
#SPJ1
fill in the blank. if dissociation of mgcl2 in water were 100%, the van`t hoff factor would be___; however, for real solutions the van`t hoff factor for mgcl2 is (greater than, less than) this value.
Answers
If dissociation of mgcl2 in water were 100%, the van`t hoff factor would be 3 (three); however, for real solutions the van`t hoff factor for mgcl2 is (greater than, less than) this value.
MgCl2 dissociates into three ions when it dissolves in water, giving it a theoretical van't Hoff factor of 3. The van't Hoff factor for MgCl2 is normally tested at a value of no more than 2.7.
The van't hoff factor for magnesium chloride is 3. The letter I stands for the Van't Hoff factor. One magnesium cation and two chloride ions make up the last three ions formed when magnesium chloride separates. Consequently, the magnesium chloride van't hoff factor is equal to 3.
A solute's impact on associated properties, such as osmotic pressure, relative vapor pressure reduction, boiling-point elevation, and freezing-point depression, is measured by the van 't Hoff factor i.
To know about the van't hoff factor
https://brainly.com/question/21854669
#SPJ4
Question 11: How does the energy of a photon emitted when the electron moves from the 3rd orbital to the 2nd orbital compare to the energy of a photon absorbed when the electron moves from the 2nd orbital to the 3rd orbital?
Answers
Answer:
Explanation:
The energy of a photon emitted when the electron moves from the 3rd orbital to the 2nd orbital is exactly same as the energy of a photon absorbed when the electron moves from the 2nd orbital to the 3rd orbital
1. Which of the following pairs of elements is most likely to share one or more pairs of electrons between
their nuclei?
a. Lithium and iodine
b. Sodium and lead
c. Copper and oxygen
d. Sulfur and chlorine
Answers
Answer:
d. Sulfur and chlorine.
Explanation:
A covalent bond is formed by two non-metals with similar electronegativities. As a consequence, they share one or more pairs of electrons between their nuclei.
Which of the following pairs of elements is most likely to share one or more pairs of electrons between their nuclei?
a. Lithium and iodine. NO. Lithium is a metal and iodine a non-metal.
b. Sodium and lead. NO. Both sodium and lead are metals.
c. Copper and oxygen. NO. Copper is a metal and oxygen is a non-metal.
d. Sulfur and chlorine. YES. Both are non-metals.
10. Iron (III) oxide is used as a source material for iron in steel manufacturing. The density ofiron (III) oxide is 5.242 g/cm'.a. If a plant needed to transport 88,915 kg of iron(III) oxide every day for operations, whatvolume of iron(III) oxide is transported (in Liters)?10a.b. If a standard, tandem axle dump truck can haul 15 tons of ore and the mine is one dayaway from the manufacturing site, how many dump trucks are needed each day?ti10b.c. Each dump truck must drive 285 miles from the source to the manufacturing site. If eachtruck gets 7.8 miles per gallon of gas and the price of gas is $3.95 per gallon, what is thefuel cost, per day, for the manufacturing plant?10c.
Answers
ANSWER
The volume of iron (III) oxide is 16962.04 Liters
STEP-BY-STEP EXPLANATION:
Given information
The density of iron (III) oxide = 5.242 g/cm^3
The mass of iron (III) oxide = 88,915 kg
Let x represents the volume in liters of iron (III) oxide
The first step is to convert the mass from kilograms to grams for unit consistency since the density is already measured in g/cm^3
Recall that, 1 kg is equivalent to 1000 g according to the standard international unit
let x represents the mass to be converted to grams
\(\begin{gathered} 1kg\text{ }\rightarrow\text{ 1000 grams} \\ 88915kg\text{ }\rightarrow\text{ xgrams} \\ \text{Cross multiply} \\ 1\cdot\text{ x = 1000 }\cdot\text{ 88915} \\ x\text{ = 88915000 grams} \end{gathered}\)From the calculation above, you will see that the mass of iron (III) oxide in grams is 88915000 grams.
The next step is to find the volume of iron (III) oxide using the below formula
\(\text{ Density = }\frac{mass}{\text{volume}}\)\(\begin{gathered} \text{Isolate volume} \\ \text{mass = density x volume} \\ \text{volume = }\frac{mass}{\text{density}} \end{gathered}\)\(\begin{gathered} \text{Volume = }\frac{88915000}{5.242} \\ \text{Volume = 16962037.39}cm^3 \end{gathered}\)The next step is to convert the cubic centimeters to liters
According to the standard international unit, 1 cm^3 is equivalent to 0.001 L
\(\begin{gathered} 1cm^3\text{ }\rightarrow\text{ 0.001L} \\ 16962307.39cm^3\text{ }\rightarrow\text{ xL} \\ \text{Cross multiply} \\ 1\cdot\text{ x = 0.001 }\cdot\text{ 1}6962307.39 \\ x\text{ = 16962.04 Liters} \end{gathered}\)Therefore, the volume of iron (III) oxide is 16962.04 Liters
Example A vegetable are chopped Example B food is broken up into simpler form during digestion which statement is correct
Answers
Both Example A and Example B are correct, but they refer to different processes. Example A refers to the physical action of chopping vegetables into smaller pieces, which can make them easier to cook and eat.
This process does not change the fundamental nature of the vegetable, but simply alters its physical properties.
Example B, on the other hand, refers to the process of digestion in the human body. During digestion, food is broken down into simpler forms, such as sugars, amino acids, and fatty acids, which can be absorbed and used by the body for energy and other functions. This process involves both physical and chemical changes, as enzymes in the digestive system break down complex molecules into simpler ones.
In summary, Example A describes a physical process that alters the size and shape of a vegetable, while Example B describes a physiological process that breaks down complex food molecules into simpler forms for use by the body. Both processes are important for preparing and consuming nutritious food.
for more such question vegetables
https://brainly.com/question/18134846
#SPJ11
2NO(g) + O₂(g) = 2NO₂(g)
AH = -112 kJ K = 0.50
The equilibrium concentrations are
[NO] = 0.31 M, [02] = 1.10 M, and
[NO2] = [?]
What is the equilibrium concentration of
NO2 at this temperature?
![2NO(g) + O(g) = 2NO(g)AH = -112 kJ K = 0.50The equilibrium concentrations are[NO] = 0.31 M, [02] = 1.10](https://i5t5.c14.e2-1.dev/h-images-qa/contents/attachments/YRGcrEmw6OtcUiE6tB5KUryscULOSlX5.png)
Answers
The equilibrium concentration of NO₂ at this temperature is approximately 0.219 M.
To determine the equilibrium concentration of NO₂, we can use the equilibrium constant expression (Kc) and the given equilibrium concentrations of NO and O₂. The equilibrium constant expression for the given reaction is:
Kc = ([NO₂]²) / ([NO]²[O₂])
We are given the equilibrium concentrations of NO and O₂ as [NO] = 0.31 M and [O₂] = 1.10 M, respectively. We need to find the equilibrium concentration of NO₂, denoted as [NO₂].
Using the given equilibrium concentrations and the equilibrium constant expression, we can rearrange the equation and solve for [NO₂]:
Kc = ([NO₂]²) / ([NO]²[O₂])
0.50 = ([NO₂]²) / ((0.31 M)²(1.10 M))
0.50 = ([NO₂]²) / (0.0961 M³)
Multiplying both sides by 0.0961 M³, we have:
0.04805 M³ = [NO₂]²
Taking the square root of both sides, we find:
[NO₂] = √(0.04805 M³)
[NO₂] ≈ 0.219 M
Therefore, the equilibrium concentration of NO₂ at this temperature is approximately 0.219 M.
It's important to note that the units of concentration (M) were used throughout the calculations, and the answer is rounded to three significant figures based on the given data.
Additionally, the negative sign of the enthalpy change (AH) indicates an exothermic reaction, and the equilibrium constant (K) of 0.50 suggests that the reaction favors the products, as the concentration of NO₂ is greater at equilibrium.
For more such questions on equilibrium concentration visit:
https://brainly.com/question/13414142
#SPJ8
Which of the following compounds displays the greatest ionic character in its bonds? A) CO 2 B) H20 C) NH3 D) NO 2 E) HF
Answers
It is observed that the electronegativity difference in the bond between the two atoms is highest in the case of H2O. Thereby, it has a greater ionic bond character.
What does the character 0% ionic mean?Pauling proposed quantifying the percentage of ionic character that a chemical link possesses. A complete ionic link would obviously have 100% ionic character, while a covalent bond with an equal share of the charge density has 0% ionic character.
How is ionic character ascertained?We examine the electronegativity of the two atoms participating in a bond in order to determine its ionic character (or polarity). The connection has a stronger ionic character the larger the difference. The placement of polar bonds in the compound's three - dimensional structure allows us to determine the polarity of the entire complex.
To know more about ionic bond visit:
https://brainly.com/question/11527546
#SPJ4
If d represents the density of a gas and k is a constant. The relationship between the rate of diffusion r, and d is ____?
Answers
The relationship between the rate of diffusion r, and d is r ∝ 1/√d.
The relationship between the rate of diffusion (r) and the density of a gas (d) can be explained using Graham's law of diffusion. According to this law, the rate of diffusion of a gas is inversely proportional to the square root of its density. Mathematically, it can be expressed as:
r ∝ 1/√d
where the symbol '∝' represents 'proportional to'. The constant of proportionality (k) can be introduced to this equation as:
r = k/√d
This equation shows that as the density of a gas increases, its rate of diffusion decreases. This is because denser gases have more molecules per unit volume and thus, they experience greater intermolecular collisions that hinder their movement. Therefore, it requires more energy for them to diffuse through a medium compared to less dense gases.
The relationship between the rate of diffusion and density is particularly important in understanding the behavior of gases in different environments. For instance, in a gas chromatography column, the rate of diffusion of a gas determines how quickly it moves through the column and separates from other components. Similarly, in the Earth's atmosphere, the rate of diffusion of greenhouse gases such as carbon dioxide affects their concentration and hence, their impact on climate change.
For more such questions on diffusion
https://brainly.com/question/29064792
#SPJ11
HOW MANY KILOWATT HOURS OF ELECTRIC ENERGY WOULD THIS REFRIGERATOR USE IN 30 DAYS. ASSUME THE REFRIGERATOR IS RUNNING 12 PER H A DAY
Answers
The electrical energy used by refrigerator in 30 days is 1,800 watt-hours or 1.8 kilowatt-hours (kWh)
What is electrical energy?Electrical energy is a form of energy that results from the movement of charged particles, such as electrons. It is a type of energy that is used to power a wide range of devices, from household appliances to large industrial machinery.
Electricity is produced when a flow of electrons is created, either by a chemical reaction, as in a battery, or by an electromagnetic induction, as in a generator.
Therefore, the electrical energy used by refrigerator in 30 days is 1,800 watt-hours or 1.8 kilowatt-hours (kWh)
To learn more about electrical energy from the given link:
https://brainly.com/question/776932
#SPJ1
At a temperature of 408K, which gad will have the highest velocity?
Answers
Answer:
1 - NO2 at 339 K
2 - Ne at 371 K
3 - H2 at 371 K
4 - H2 at 425 K
Explanation:
Kinetic Energy is directly related to temperature; the higher the temperature the higher the kinetic energy. Kinetic energy is also equal to 12m⋅v2, so if we want a high velocity we want high temp and low mass. So let's list out approximate masses:
m(H2)≈2
m(NO2)≈46
m(Ne)≈20
So we have NO2 at 339 K, the lowest temperature out of the mix, and the highest mass out of the mix, so this is moving the slowest.
In contrast, we have H2 at 425 K, the highest temperature out of the mix, and the lowest mass out of the mix, so this is moving the fastest.
Now we have Ne and H2 at 371 K, since they are at the same temperature they have the same kinetic energy. But H2 is lighter than Ne so it must be faster. To quantify this mathematically, let's assume (this is wrong but just as an assumption for an example) KE at 371 K is 100:
100=12⋅m⋅v2
200=m⋅v2
√200m=v
So H2 is about v=10 and Ne is about v=√10≈3
So the order to recap is:
1 - NO2 at 339 K
2 - Ne at 371 K
3 - H2 at 371 K
4 - H2 at 425 K
Hope that makes it clearer!
Which is a product of nuclear fusion?
A a nucleus that has a greater mass number than the starting materials
B two nuclei that are roughly half the mass of the starting materials
C a nucleus that has the same atomic number as the starting materials
D two nuclei that have greater atomic numbers than the starting materials
Answers
Answer:
A. a nucleus that has a greater mass number than the starting materials
Explanation:
The statement is "a nucleus that has a greater mass number than the starting materials."
What is nuclear fusion?Nuclear fusion occurs when two or more atomic nuclei unite to generate one or more new atomic nuclei and subatomic particles.
Two light nuclei fuse to form a single heavy nucleus in a fusion reaction. Because the overall mass of the resulting single nucleus is smaller than the mass of the two initial nuclei, the process releases energy.
Hence, the correct option is A
To more know about nuclear fusion, here,
https://brainly.com/question/12701636
#SPF2
Once the roller coaster train gets closer to the bottom of the hill, its kinetic energy increases to 1,100 J, and its potential energy decreases to
Answers
Once the roller coaster train gets closer to the bottom of the hill, its kinetic energy increases to 1,100 J, and its potential energy decreases to zero.
What is potential energy?Potential energy is a type of energy that is stored and depends upon the relative height of system. If its height is increased, the potential energy is also increases while on the other hand, if the body comes to the surface of the earth, its potential energy will be zero due to no height of the object. We know that potential energy is equals to product of mass, gravity and height of an object.
So we can conclude that if the roller coaster train gets closer to the bottom of the hill, its kinetic energy increases to 1,100 J, and its potential energy decreases to zero.
A student uses 200 grams of water at a temperature of 60 °C to prepare a saturated solution of potassium chloride , KCI. Identify the solute in this solution.
1. H2O(l)
2. KCl (aq)
3.K + (aq)
4.KCl(s)
Answers
Answer:
4. KCl(s)
Explanation:
KCl is an ionic salt that dissolves in water to form a KCl aqueous solution.
A solution is defined as the homogeneous mixture of one or more solutes dissolved in a solvent. Here in the saturated solution of potassium chloride, the solute is KCl. The correct option is 4.
What is a solute?A solute is defined as the substance which is dissolved in a solution. In a solution the amount of the solute present is always smaller than the amount of the solvent. For example in a salt solution, salt dissolves in water and therefore salt is the solute.
The particles of the solute present in a solution cannot be seen by our eye. The solute from a solution is not possible to separate by filtration. In an unsaturated solution, the concentration of the solute is much lower than that of the concentration of the solvent.
A solution is a combination of the solute and the solvent.
Thus the correct option is 4 - KCl.
To know more about solute, visit;
https://brainly.com/question/13812915
#SPJ2
Which of the following choices is NOT an example of a colligative property?A. Vapor pressure loweringB. Freezing point depressionC. Boiling point elevationD. Melting point acceleration
Answers
Explanation: Collagative properties are characteristics of a solution that is dependent on the ratio of solute particles to to solvent particles.
Answer: D) Melting point acceleration.
What characteristic of a light wave in a medium determines the index of refraction of that
medium?
Answers
Answer:
The refractive index can be seen as the factor by which the speed and the wavelength of the radiation are reduced with respect to their vacuum values: the speed of light in a medium is v = c/n, and similarly the wavelength in that medium is λ = λ0/n, where λ0 is the wavelength of that light in vacuum.
Explanation:
Explanation: i need points