The stability and inert nature of noble gases is caused by:
a full d sublevel
a full f sublevel
a full p and a full d sublevel
a full s and a full p sublevel
Answers
Answer: a full s and a full p sublevel
Explanation: "noble gases. They are an especially important group of the periodic table because they are almost completely unreactive, due to their completely filled outermost s and p sublevels."
Related Questions
2(t. A gas sample is held at constant pressure. The gas occupies 3.62 L of volume when the temperature is 21.6"C. Determine thetemperature at which the volume of the gas is 3.45 L.a) 309 K b) 281 K e) 20,6 K d) 294 K e) 326 K
Answers
They tell us that the pressure of the gas is constant and the temperature and volume vary. If we assume that the gas behaves like an ideal gas, we can apply Charles's law, which tells us:
\(\frac{V_1}{T_1}=\frac{V_2}{T_2}\)where,
V1 is the initial volume, 3.62L
T1 is the initial temperature, 21.6°C=294.75K
V2 is the final volume, 3.45L
T2 is the final temperature, in Kelvin
Now, we clear T2 and replace the known data:
\(T_2=V_2\times\frac{T_1}{V_1}\)\(T_2=3.45L\times\frac{294.75K}{3.62L}=281K\)The temperature at which the volume of the gas is 3.45 L will be 281K
Answer. b) 281K
The specific heat capacity of methane gas is 2.20 J/g°C. How many joules of heat are needed to raise the temperature of 5.00 g of methane from 22.0°C to 57.0°C?
a-79.5 J
b-385J
c-869 J
d-0.0126 J
Answers
Answer:
b - 385 j
Explanation:
is density defined as the volume in a given mass orthe mass in a given volume?
Answers
Answer:
mass in a given volume
Explanation:
Density is the mass in a given volume or mass per unit volume of a substance.
Mass is defined by the amount of matter present in a substance.
Volume is the space occupied by a body.
Density is the mass divided by the volumeMathematically;
Density = \(\frac{mass}{volume}\)
what pressure would it take to compress 250. l of helium gas initially at 1.00 atm into a 2.00 l tank at constant temperature?express your answer with the appropriate units.
Answers
1.25atm is the pressure required to compress 250l of helium gas.
What is Helium gas?
The chemical element helium has the atomic number 2 and the symbol He. It is the first member of the noble gas group in the periodic table and is a colourless, odourless, tasteless, non-toxic, inert, monatomic gas. Of all the elements, it has the lowest melting and boiling points.
What is pressure?
The force delivered perpendicularly to an object's surface per unit area across which that force is dispersed is known as pressure.
Calculations:
Here the temperature is constant.
Given
P1 = 1atm
V1 = 250L
P2 = ?
V2 = 2 L
Hence,
P1V1 = P2V2
P2 = P1V1/V2
P2 = 250L × 1atm/200L = 1.25atm.
Hence, 1.25atm is the pressure required to compress 250l of helium gas.
To know more about Helium gas, check out:
https://brainly.com/question/28302901
#SPJ4
Please help with this

Answers
1. Double-Replacement reactions
2. Decomposition
3. Combustion
4. Syntesis
5. Single replacement
Further explanationGiven
Chemical equations
Required
Type of reaction
Solution
1. 2AgNO₃ + MgCl₂ ⇒ 2AgCl + Mg(NO₃)₂
Double-Replacement reactions. Happens if there is an ion exchange between two ion compounds in the reactant
2. 2KBr⇒2K +Br₂
Decomposition
Single compound breaks down in to 2 or more products
3. C₃H₈ + 5O₂ ⇒ 4H₂O + 3CO₂
Combustion
Hydrocarbon and Oxygen reaction and form water and carbon dioxide
4. NaO + H₂O ⇒ NaOH
Syntesis
2 or more reactants combine to form a single product
5. Zn + CuCl₂ ⇒ZnCl₂ + Cu
Single replacement
One element replaces another element from a compound
How many moles of Ar gas are
present in a container with a
volume of 78.4 L at STP?
Answers
1 mole of a gas at STP occupies 22.4 L volume
Now the volume is given =78.4 therefore,
No. of moles of gas = 78.4 ÷ 22.4 = 3.5 moles
I hope it helps you~
Infer what the term “biogeochemical cycles” means. How do you think the term relates to a food chain?
Answers
Answer: Biogeochemical cycles are usually described with box models.
Explanation: Matter flows through trophic levels and elements are recycled among ecosystems using biogeochemical cycles. As nutrients move through ecosystems, the compounds they form are usually transformed.
Substances that can be drawn into wires without breaking are
Answers
Some examples are gold, silver, copper, etc
In the diagram, which organism is a primary consumer?
clover
owl
rabbit
hawk
Answers
Answer:
Rabbit they eat plants for energy
Explanation:
Answer:
Clover
Explanation:
A clover is the only organism that makes its own food, usually from the sun. The other three need to eat to get their energy. Rabbits eat plants, owls eat insects, and hawks eat other types of animals such as fish.
A 4.80g piece of magnesium displaces 2.76 mL of water when it is placed in a graduated cylinder. What is the density of magnesium?
Answers
Answer:
6
What do AIA, SAG, AFP and AMA all stand for? Select four options.
American Institute of Architects
American Society of Mechanical Engineers
American Medical Association
Screen Actors Guild
Association for Finance Professionals
Professional Colleges
Balance each equation
11. KOH(aq) + HBr(aq) → KBr + H20(0)
12. H2CO3 (aq) → + CO2 (g) H20
13. Na (s) + O2 (g) → Na2O (s)
14. Al(OH)3(aq) + H2CO3(aq) → Al2(CO3)3 + H2O
15. Al (s) + S8 (s) → AIS (s)
16. Cs (s) + N2 (g) → Cs3N (s)
17. Mg (s) + Cl2 (g) → MgCl2 (s)
18. Rb (s) + RbNO3 → Rb20 (s) + N2 (g)
19. C.H. (0) + O2 (g) → CO2 (g) + H20 (g)
20. N2 (g) + H2 (g) → NH3(g)
Answers
Answer:
WHats how same question no very good inglish pls
Explanation:
Hydrazine (N_2H_4) decomposes to produce N_2 and NH_3. How many molecules of NH_3 will be formed if 60 molecules of N_2H_4 decompose into N_2 and NH_3
Answers
80 molecules of \(NH_{3}\) will be formed if 60 molecules of \(N_{2}H_{4}\) decomposes into \(N_{2}\) and \(NH_{3}\)
What are molecules?The smallest particle of a substance has all of the physical and chemical properties of that substance. Molecules are made up of one or more atoms.
Just balance the reaction:
\(3N_{2}H_{4}\to N_{2}+4NH_{3}\)
Now you can see that every 3 molecules of \(N_{2}H_{4}\)4 molecules of \(NH_{3}\).
For 60molecules of \(N_{2}H_{4}\) we scale up these numbers by a factor of 60/3 = 20.
Therefore 4x20 = 80 molecules of \(NH_{3}\) are formed.
Learn more about molecules here:
https://brainly.com/question/19922822
#SPJ1
why do living things need water, food, and space
Answers
living thing need water to survive
and food to get energy and perform life processes.
and living organism need space to gets the energy and materials it needs
A compound consists of 3 moles of carbon and 4 moles of hydrogen. What is the empirical formula of this compound?
Group of answer choices
CH4
C3H3
C3H4
C4H3
Answers
Answer:thanks for the points
Explanation:
The half-life of iodine-131 is 8 days. How many milligrams of iodine-131
remain after 24 days if the original amount was 4.00 mg?
A. 1.33 mg
B. 0.667 mg
C. 0.500 mg
D. 0.444 mg
Answers
Answer:
The correct answer is - C. 0.500 mg.
Explanation:
In this case, the half-life of the iodine-131 is 8 days and the initial amount is given 4 mg. According to this after every 8 days, half of the initial value of the iodine-131 remains only.
8 days or 1st half-life = A(i)* 1/2
16 days or two half-life = A(i)* 1/4
24 days or three half-life = A(i)* 1/8
and the remaining amount A would be
= A(i)*1/2^n
= 4 * 1/2^3
= 4 *1/8
= 0.500 mg
1/ Hydra, a type of cnidarian, have equal survival rates through life because they are equally fit at all stages. Hydra are an example of an organism with a type ______ survivorship curve. Select one: a. 0 b. I c. II d. III e. IV 2/ What is different about how energy moves through an ecosystem compared to how chemicals move through the ecosystem? 3/ Tropical rain forest soils are usually Select one: a. nutrient-rich. b. low in organic matter. c. nutrient-poor and low in organic matter. d. nutrient-rich and low in organic matter. e. nutrient-poor.
Answers
Hydra, a type of cnidarian, have equal survival rates through life because they are equally fit at all stages. Hydra are an example of an organism with a type I survivorship curve. Thus, the correct option is b. II.
Differences between how energy moves through an ecosystem compared to how chemicals move through the ecosystem. The fundamental difference between how energy moves through an ecosystem compared to how chemicals move through the ecosystem is that energy can not be recycled. Energy is obtained from the sun and is stored in organic molecules, and it flows through an ecosystem through metabolic processes. However, the number of chemicals like carbon, oxygen, nitrogen, water, and phosphorus remains constant in an ecosystem.
Tropical rainforest soils are usually nutrient-poor and low in organic matter. Therefore, option c. nutrient-poor and low in organic matter is the correct answer. The soil in the tropical rainforest is nutrient-poor because heavy rainfall washes away the nutrients, and the soil contains a high level of aluminum and iron, which are toxic to plants. Due to these reasons, there is slow decomposition of organic matter that makes the soil low in organic matter.
Thus, the correct option is b.
To learn more about ecosystem check the link below-
https://brainly.com/question/842527
#SPJ11
Which one of the following is the highest temperature? A) 38 °C B) 96 °F C) 302 K D) none of the above E) the freezing point of water
Answers
Answer:
The highest temperature is 302K
Explanation:
The answer is C
The highest temperature among the given options is 302 K.
To determine the highest temperature among the given options, we need to convert them to a common scale and compare.
Option A) 38 °C: This is a temperature in Celsius.
Option B) 96 °F: This is a temperature in Fahrenheit.
Option C) 302 K: This is a temperature in Kelvin.
Option D) None of the above: This option does not provide a specific temperature.
Option E) The freezing point of water: This is 0 °C, 32 °F, and 273.15 K.
Comparing the given options, we can see that 302 K is the highest temperature among them.
Learn more:About highest temperature here:
https://brainly.com/question/14863386
#SPJ11
0.0325 m = [?] cm
pls help me asap
Answers
Answer:
The answer would be 3.25 cm
Explanation:
Not needed.
Which has the strongest intermolecular forces?
A B C or D
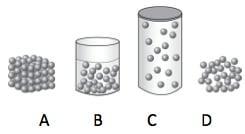
Answers
Answer:
A
Explanation:
What’s a physical change
Answers
Answer:Physical changes are changes affecting the form of a chemical substance, but not its chemical composition. Physical changes are used to separate mixtures into their component compounds, but can not usually be used to separate compounds into chemical elements or simpler compounds.
Explanation:
cyclopropyl chloride has been prepared by the free-radical chlorination of cyclopropane. draw the products for each equation of the stepwise mechanism for this reaction. be sure to include lone pair(s) of electrons where necessary.
Answers
Free radicals have chlorinated cyclopropane to create cyclopropyl chloride.
C3H5Cl : C3H5+ + Cl - Cl
Chlorination is the process of adding chlorine to potable water to kill parasites, viruses, and other microorganisms. There are several methods that can be used to ensure that drinking water has safe chlorine levels. Chlorine in water at low concentrations helps stop the spread of diseases that are transmitted by water and is safe to drink or ingest. Your water provider conducts regular water quality testing in order to supply you with safe drinking water. It is possible for lakes and wells to get contaminated with microorganisms that really can make people sick. Additionally, water can become contaminated with bacteria as it travels through miles of tubing to reach a neighborhood. To prevent infection with germs, water firms often add a disinfectant—typically chlorine or chloramine—that kills disorder microorganisms like Salmonella.
To know more about chlorination visit :
https://brainly.com/question/20834469
#SPJ4
which molecule can diffuse from the digestive tract into
Answers
Glucose can diffuse from the digestive tract into the human bloodstream without first being digested.
Glucose is the most typical monosaccharide, or kind of carbohydrate. Most plants and algae use solar energy to synthesize glucose during photosynthesis from water and carbon dioxide. This glucose is then used to make cellulose, the most common carbohydrate in nature, in the cell walls of the plants and algae. The most important source of energy for all species' energy metabolism is glucose. For use in metabolism, glucose is stored as a polymer, mostly as starch and amylopectin in plants and glycogen in mammals. Animals' blood contains glucose as blood sugar.
Learn more about Glucose
brainly.com/question/2396657
#SPJ4
Compare Fossils X and Y during tension. What happens to each?
Your answer
Answers
Without specific information about the characteristics or nature of Fossils X and Y, it is difficult to provide a direct comparison of their behavior during tension.
However, in general, when objects experience tension, certain behaviors can be observed:
Elastic deformation: When subjected to tension, some materials, such as elastic materials like rubber bands or springs, exhibit elastic deformation. This means that they can stretch or deform under tension but return to their original shape once the tension is released. If Fossil X or Y is made of an elastic material, it may stretch under tension and then return to its original shape once the tension is removed.
Plastic deformation: Other materials, like certain metals or plastics, may undergo plastic deformation under tension. Plastic deformation involves a permanent change in shape or structure, even after the tension is released. If Fossil X or Y is made of a material that exhibits plastic deformation, it may experience permanent stretching or deformation when subjected to tension.
Failure or fracture: If Fossil X or Y is unable to withstand the applied tension, it may eventually fail or fracture. This can occur if the material is weak or if the applied tension exceeds its strength. Failure can result in the complete separation of the fossil into multiple pieces or the breaking of bonds within the material.
It is important to note that without specific details about the characteristics and composition of Fossils X and Y, it is challenging to provide a precise comparison of their behaviors during tension. The properties and behavior of the materials that make up the fossils will determine their response to tension.
To know more about nature of Fossils click this link -
brainly.com/question/1685421
#SPJ11
The texture of many foods is determined by the physical state of the lipid phase. Which one of these statements is NOT true? a. The solid fat content versus temperature profile plays an important role in determining the texture
b. The morphology of the crystals formed plays an important role in determining the texture
c. The texture of foods containing partially crystalline lipids can be described as "plastic"
d. The polymorphic form of fat crystals is in a glassy state
Answers
d. The polymorphic form of fat crystals is in a glassy state is NOT true.
The statement that the polymorphic form of fat crystals is in a glassy state is not true. Polymorphism refers to the ability of a substance to exist in multiple crystal structures or forms. Lipids, including fats, can exhibit polymorphism, meaning they can crystallize in different arrangements or crystal forms.
When it comes to the texture of foods containing lipids, the polymorphic form of fat crystals does play a significant role. The specific crystal form and arrangement of the lipids can affect the texture of the food, influencing factors such as mouthfeel, creaminess, and stability.
The solid fat content versus temperature profile is an essential factor in determining texture, as stated in option a. The morphology of the crystals formed, as mentioned in option b, also plays a crucial role in texture. Option c is true as well, as foods containing partially crystalline lipids can exhibit a "plastic" texture.
However, option d is not accurate because the polymorphic form of fat crystals can exist in various states, including crystalline and semi-crystalline states, but not in a glassy state. Glassy states are typically associated with amorphous materials rather than crystalline structures.
To learn more about lipids, here
https://brainly.com/question/14915606
#SPJ4
A sample of water has a mass of 100.0 g. Calculate the amount of heat required to change the sample from ice at -45.0°C to liquid water at 75.0°C. Use the chart to complete the multiple steps required to arrive at the final answer. Type in your answers below using 3 digits.
q1 =
kJ
q2 =
kJ
q3 =
kJ
qtot =
kJ
ΔT(K) Phase
Change Constant Formula
-45.0➝0.00 Warm solid Cp, solid = 2.093
J/g ⋅ K q1 = cmΔT
0.00 Melt solid ΔHfusion = 40.7
kJ/mol q2 = nΔHfus
0.00➝75.0 Warm solid Cp, liquid = 4.184
J/g ⋅ K q3 = cmΔT
-45.0➝75.0 Sol➝liq qtot = q1 + q2 + q3
Answers
The amount of heat required to change the sample from ice at -45.0°C to liquid water at 75.0°C is 74305 J.
What is the amount of heat required to change the sample from ice at -45.0°C to liquid water at 75.0°C?The amount of heat required to change the sample from ice at -45.0°C to liquid water at 75.0°C is calculated as follows:
Heat to change from ice at -45.0 °C to ice at 0°C, Q₁ = mc₁ΔT
The heat required to change ice at 0°C to water at 0 °C, Q₂ = mL
Heat required to change water at 0°C to water at 75 °C, Q₃ = mc₂ΔT
Total heat required = Q₁ + Q₂ + Q₃
where;
m is mass of water = 100.0 gc₁ is the specific heat of ice = 2.09 J/g·°C;ΔT is the temperature changeL is the latent heat of fusion of ice = 334 J/gc₂ is the specific heat of water = 4.2 J/g°CQ₁ = 100 * 2.09 * {0 - (-45)
Q₁ = 9405 J
Q₂ = 100 * 334
Q₂ = 33400 J
Q₃ = 100 * 4.2 * (75 - 0)
Q₃ = 31500 J
Total heat = 9405 J + 33400 J + 31500 J
Total heat required = 74305 J
Learn more about specific heat at: https://brainly.com/question/27991746
#SPJ1
The gas left in a used aerosol can is at a pressure of 3.2 atm at 38°C. If this can is thrown into a fire, what is the internal pressure of the gas when its temperature reaches 433°C?
Answers
Therefore, the internal pressure of the gas in the can when its temperature reaches 433°C is approximately 52.9 atm.
What is pressure?In physics, pressure is defined as the force applied per unit area. It is a scalar quantity and is expressed in units such as pascals (Pa), pounds per square inch (psi), or atmospheres (atm). Pressure can be exerted by a gas or a liquid, and it is related to the density and temperature of the substance. It is an important concept in many areas of science and engineering, including fluid dynamics, thermodynamics, and materials science.
Here,
To solve this problem, we can use the combined gas law, which relates the pressure, volume, and temperature of a gas:
P1V1/T1 = P2V2/T
where P1, V1, and T1 are the initial pressure, volume, and temperature, and P2, V2, and T2 are the final pressure, volume, and temperature.
We can assume that the volume of the can is constant, so V1 = V2. We also need to convert the temperatures to Kelvin by adding 273.15 to them.
Plugging in the given values, we get:
(3.2 atm)(V) / (38°C + 273.15) = P2(V) / (433°C + 273.15)
Simplifying and solving for P2, we get:
P2 = (3.2 atm)(433°C + 273.15) / (38°C + 273.15)
= 52.9 atm
To know more about pressure,
https://brainly.com/question/18431008
#SPJ1
water dissolves salts because it: select one: a. is hydrophobic, and salts are also hydrophobic. b. forms covalent bonds with the atoms of the salt crystal. c. has partial positive and negative charges. d. evaporates quickly at room temperature.
Answers
Salts get dissolved in water because it has partial positive and negative charges. So option (c) is correct.
Why does salt dissolves in water?The water molecules pull the Na and Cl ions apart while breaking the ionic bond which held them together. After the salt ions are pulled apart, they get surrounded by water molecules. The salt dissolves to form a homogeneous solution.
The slightly positive portion of sodium is attracted to the slightly negative portion of oxygen on the water molecule. At the same time, the slightly electronegative chlorine moieties of NaCl are attracted to the slightly electropositive hydrogen moieties of water.In either case, no true bond is formed, the stronger covalent bonds of water (also commonly held by hydrogen bonds between water molecules) win, NaCl gets pulled apart, resulting in dissociation of Na+ and Cl- ions with the Na+ and Cl- ions setting loosely in place between the intact H₂O molecules. NaCl is then dissolved.Salts are ionic and are expected to dissolve in water because water itself is polar. Therefore, ionic salts are expected to dissolve in polar solvents.
To know more about dissolution of salts in water visit:
https://brainly.com/question/14515128
#SPJ4
help me please it's due tomorrow

Answers
After selecting the hypothesis, the next step is to design an experiment to test the hypothesis.
For example, our hypothesis is "deforestation causes soil erosion".
A hypothesis should define two variables that may exhibit a relationship between them. Scientists define which variable will be the independent variable and the dependent variable.In the above example, independent variable is deforestation and dependent variable is soil erosion.Confounding variables are unwanted variables that can affect the dependent variable of an experiment. Thus, it eventually also affect the overall data and results of the experiment. For example, possible confounding variables here could be different materials in the soil.Eliminating confounding variables helps us know for sure that changes in the dependent variable are caused solely by the independent variable.Learn more about variables here:
brainly.com/question/17344045
#SPJ9
what rule/principle states that electrons fill orbitals from lowest energy to highest enegery?
Answers
Answer:
The Aufbau Principle
Explanation:
In the ground state of an atom or ion, electrons fill atomic orbitals of the lowest available energy level before occupying higher-energy levels.
Answer:
Aufbau principle
Explanation:
edge 2021
How many milliliters of 0. 250M NaOH is required to neutralize 30. 4mL of 0. 152M HCl?
Answers
Approximately 18.4832 mL of 0.250 M NaOH is required to neutralize 30.4 mL of 0.152 M HCl.
To determine the volume of 0.250 M NaOH required to neutralize 30.4 mL of 0.152 M HCl, we can use the concept of stoichiometry and the balanced chemical equation for the neutralization reaction between NaOH and HCl:
NaOH + HCl -> NaCl + H2O
From the balanced equation, we can see that the stoichiometric ratio between NaOH and HCl is 1:1. This means that for every mole of NaOH, we require an equal number of moles of HCl to neutralize.
First, let's calculate the number of moles of HCl present in the given volume:
Moles of HCl = concentration of HCl * volume of HCl
= 0.152 M * 30.4 mL
= 4.6208 mmol (millimoles)
Since the stoichiometric ratio is 1:1, the number of moles of NaOH required to neutralize the HCl is also 4.6208 mmol.
Now, let's calculate the volume of 0.250 M NaOH needed to contain 4.6208 mmol:
Volume of NaOH = (moles of NaOH) / (concentration of NaOH)
= 4.6208 mmol / 0.250 M
= 18.4832 mL
Therefore, approximately 18.4832 mL of 0.250 M NaOH is required to neutralize 30.4 mL of 0.152 M HCl.
learn more about NaOH here
https://brainly.com/question/20573731
#SPJ11