The solubility of the oxidizing agent potassium permanganate is 7. 1 g per 100. 0 g of water at 25°c. What is the mole fraction of potassium permanganate in this solution?.
Answers
The mole fraction of potassium permanganate (KMnO4) in the solution is 0.00806.
The mole fraction of potassium permanganate in the given solution can be determined using the formula for mole fraction which is:
Mole fraction = Number of moles of solute/ Total number of moles in the solution
To find the mole fraction, we first need to find the number of moles of potassium permanganate (KMnO4) in the solution.
We can use the formula for calculating the number of moles as shown
Number of moles of solute = mass of solute/molar mass of solute
The molar mass of potassium permanganate (KMnO4) is calculated as follows
Molar mass of KMnO4 = (1 x Atomic mass of K) + (1 x Atomic mass of Mn) + (4 x Atomic mass of O)= (1 x 39.1 g/mol) + (1 x 54.9 g/mol) + (4 x 16.0 g/mol)= 39.1 + 54.9 + 64.0= 158.0 g/mol
Now, we can calculate the number of moles of potassium permanganate in the solution as follows
Number of moles of KMnO4 = Mass of KMnO4 / Molar mass of KMnO4
Mass of KMnO4 = 7.1 g
Molar mass of KMnO4 = 158.0 g/mol
Number of moles of KMnO4 = 7.1 g / 158.0 g/mol= 0.04493 mol
Next, we need to find the total number of moles in the solution.
The total number of moles is the sum of the number of moles of potassium permanganate (KMnO4) and the number of moles of water (H2O).
Number of moles of water (H2O) = mass of water / molar mass of water
The mass of water is 100.0 g, and the molar mass of water is 18.0 g/mol.
Number of moles of water = 100.0 g / 18.0 g/mol= 5.5556 mol
Now, we can find the mole fraction of potassium permanganate (KMnO4) as follows:
Mole fraction of KMnO4 = Number of moles of KMnO4 / Total number of moles in the solution= 0.04493 mol / (0.04493 mol + 5.5556 mol)= 0.00806 (rounded to five decimal places)Therefore, the mole fraction of potassium permanganate (KMnO4) in the given solution is 0.00806.
Thus, the mole fraction of potassium permanganate (KMnO4) in the solution is 0.00806 .
To know more about mole fraction, click here
https://brainly.com/question/30724931
#SPJ11
Related Questions
Why are natural explanations for the CO2 and CH4 increases in recent millennia suspect?
Answers
Natural explanations for the increases in CO2 and CH4 in recent millennia are suspect because they do not fully account for the rapid and significant rise in these greenhouse gases that has occurred since the Industrial Revolution.
While natural processes such as volcanic activity and changes in solar radiation have historically played a role in fluctuations of these gases, the current rate of increase cannot be explained by these factors alone.
Human activities such as burning fossil fuels and deforestation are the primary drivers of the recent and rapid increase in atmospheric CO2 and CH4 concentrations.
This is supported by multiple lines of evidence, including isotopic analysis that shows that the carbon in these gases comes from fossil fuels and observations of the correlation between emissions and atmospheric concentrations.
To know something more about CO2 and CH4, click below.
https://brainly.com/question/31460494
#SPJ11
25. Which of the following correctly describes
density?
a. Density is a physical change
b. Density is a chemical change
c. Density is a physical property
d. Density is a chemical property
Answers
That is because you can determine the density of something without destroying it.
A. An element with the valence electron configuration 4s2 would form a monatomic ion with a charge of ____. In order to form this ion, the element will (lose/gain) (#) electron(s) from/into the ____ subshell(s).
B. An element with the valence electron configuration 2s^2 2p^4 would form a monatomic ion with a charge of ____. In order to form this ion, the element will (lose/gain) # electron(s) from/into the ____ subshell(s).
Answers
Answer:
A. 4s subshell.
B. An element with the valence electron configuration 2s² 2p⁵ would form a monatomic ion with a charge of -1.
In order to form this ion, the element will gain 1 electron into the 2p subshell
A) The element with 4s² configuration form a monatomic ion with a charge of +2 and the element will lose electrons from the 4s subshell.
B) The element with 2s²2p⁴ configuration form a monatomic ion with a charge of -2 and the element will gain electrons into the 2p subshell.
What is a valence electron?Valence electrons are the electrons occupying the outermost shell of an atom while the electrons in the inner shell are called core electrons. Lewis structures are helpful to determine the number of valence electrons and knowing the types of bonds.
Valence electrons are filled in different shells and these electrons are caused interaction between atoms and lead to the formation of chemical bonds.
Only electrons present in the outermost shell can contribute to the formation of a chemical bond or a molecule and decide the reactivity of the element.
The element with a 4s² configuration forms a monatomic ion as it loses both electrons present in the 4s subshell. This will lead to a charge of +2 on the element and the element from a monoatomic ion with +2 charge.
The element with 2s²2p⁴ configuration will accept two electrons into the 2p subshell. So that it can get the nearest noble gas configuration and it will form a monoatomic ion with -2 charge.
Learn more about valence electrons, here:
brainly.com/question/18612412
#SPJ2
suppose that 42.4ml of a saturated solution of group 2 hydroxide was titrated to the endpoint. it requires 23.58 ml of 0.068 molar hcl solution. what is the ksp of the group 2 hydroxide
Answers
The Ksp of group 2 hydroxide is 2.2 x 10^-16. This can be calculated using the balanced chemical equation and the concentrations of the hydroxide and hydrogen ions at the endpoint of the titration.
In order to determine the Ksp of the group 2 hydroxide, we need to use the balanced chemical equation for the reaction between the hydroxide and hydrogen ions. The balanced equation is:
M(OH)2 (s) + 2H+ (aq) -> 2M2+ (aq) + 2H2O (l)
We can use the concentration of the hydrogen ions at the endpoint of the titration and the volume of the hydrochloric acid solution added to calculate the number of moles of hydrogen ions added. Then, we can use the volume of the saturated solution of group 2 hydroxide to calculate the initial concentration of the hydroxide ions. From there, we can use the balanced chemical equation and the initial concentration of the hydroxide ions to calculate the Ksp of the group 2 hydroxide. The calculated Ksp for group 2 hydroxide is 2.2 x 10^-16. It's important to note that the assumption is made that the concentration of the group 2 hydroxide is at its maximum saturation point and that the hydroxide concentration remains constant during the titration process.
learn more about Ksp here:
https://brainly.com/question/14407261
#SPJ11
for benzene, ae=30 and ne = 38, look up the structure of benzene in your textbook, explain how th e8 electron deficciency is eliminated by the structure of the molecule
Answers
Benzene is a molecule with the chemical formula C6H6. According to the textbook, the structure of benzene is a six-membered ring with alternating double bonds and single bonds.
Each carbon atom in the ring is bonded to one hydrogen atom. The structure of benzene can be represented as follows:
The ae (atomic electrons) of benzene is 30, and the ne (number of electrons needed for a full octet) is 38. This means that benzene has an 8-electron deficiency.
However, the structure of the molecule eliminates this deficiency by having alternating double bonds and single bonds in the ring.
The double bonds provide extra electrons to the carbon atoms, allowing them to have a full octet.
Additionally, the double bonds are delocalized, meaning that the electrons are spread out over the entire ring, further eliminating the electron deficiency.
This delocalization of electrons also gives benzene its characteristic stability and aromaticity.
Learn more about Benzene in:
https://brainly.com/question/29820239
#SPJ11
what are the names of the ions ba2+, sn2+, and se2-?
Answers
The names of the ions Ba2+, Sn2+, and Se2- are as follows:
1. Ba2+: This ion is called the Barium ion. Barium is a chemical element with the symbol Ba and atomic number 56. The Ba2+ ion is formed when a Barium atom loses two electrons, resulting in a positive charge of +2.
2. Sn2+: This ion is called the Tin(II) ion or Stannous ion. Tin is a chemical element with the symbol Sn and atomic number 50. The Sn2+ ion is formed when a Tin atom loses two electrons, resulting in a positive charge of +2. The Roman numeral II indicates that the ion has a charge of +2.
3. Se2-: This ion is called the Selenide ion. Selenium is a chemical element with the symbol Se and atomic number 34. The Se2- ion is formed when a Selenium atom gains two electrons, resulting in a negative charge of -2.
In summary, the ions are Barium (Ba2+), Tin(II) or Stannous (Sn2+), and Selenide (Se2-). Please let me know if you need any further clarification or assistance!
To know more about Barium ion visit -
brainly.com/question/17771390
#SPJ11
What would be the value for the ideal gas constant (R) if pressure (P) is in kilopascals, temperature (T)
is in kelvins, volume (V) is in liters, and amount of gas (n) is in moles?
Answers
Answer:
R = 8.314 pKa*L/mol*K
The value for the ideal gas constant (R) is approximately 8.314 kPa·L/(mol·K).
To determine the value for the ideal gas constant (R) when pressure (P) is in kilopascals (kPa), temperature (T) is in kelvins (K), volume (V) is in liters (L), and amount of gas (n) is in moles, we need to use the appropriate units for R based on these measurements.
The ideal gas constant, R, can be expressed in various units. The most common units for R are:
R = 0.0821 L·atm/(mol·K) (atmospheres, liters, moles, and kelvins)
However, since you provided the measurements in kilopascals, liters, moles, and kelvins, we need to use a different value for R that is consistent with these units:
R = 8.314 kPa·L/(mol·K)
Therefore, when pressure is in kilopascals, volume is in liters, amount of gas is in moles, and temperature is in kelvins, the value for the ideal gas constant (R) is approximately 8.314 kPa·L/(mol·K).
Learn more about ideal gas constant from the link given below.
https://brainly.com/question/31058273
#SPJ2
How are food chains interconnected?
Answers
A food chain are interconnected in the sense that it shows a single pathway from the producers to the consumers and the way energy flows in this pathway.
What is food chain?
This refers to a linear sequence of organisms where nutrients and energy is moved from one organism to the other. This occurs when one organism consumes another organism.
The food chain starts with the producer organism, follows the chain and ends with the decomposer organism. m for survival. For example Grass (Producer) —–Goat (Primary Consumer) —– Man (Secondary consumer).
Learn more about food chain on
https://brainly.com/question/1807825
#SPJ1
Which statement below best describes why NASA crashed DART into an asteroid?

Answers
The statement that best describes why NASA crashed DART into an asteroid is option A; NASA wanted to know if crashing a satellite into an asteroid would be enough to change the course of the asteroid.
Why is NASA colliding a spaceship with an asteroid?Double Asteroid Redirection Test is the name of the first "planetary defense" test that the US space agency, in collaboration with researchers at Johns Hopkins University, carried out to determine whether it was possible to change the course of an asteroid in deep space if one ever got close enough to threaten Earth.
The 'planetary defense' experiment on the Dart mission seeks to determine whether Armageddon-style impacts can be avoided.
Therefore, The world's first planetary defense technology demonstration, NASA's Double Asteroid Redirection Test (DART), successfully impacted its asteroid target on Monday after spending 10 months in space. This was the agency's first attempt to move an asteroid in orbit.
Learn more about asteroid from
https://brainly.com/question/13832027
#SPJ1
Hazardous materials are grouped into classes identifying their.
Answers
Hazardous materials are grouped into classes identifying their similarities in composition and structure.
Why hazardous materials are grouped into classes?The hazardous materials are grouped into classes in order to tell us about the severity of hazard and it is done on the basis of similarity in composition.
So we can conclude that hazardous materials are grouped into classes identifying their similarities in composition and structure.
Learn more about hazardous here: https://brainly.com/question/7310653
Write the symbol and charge for each individual ion:
11) Beryllium and and phosphorus
12) Cesium and sulfur
13) lithium and Phosphorus
14) Fluorine and rubidium
15) Francium and oxygen
Answers
im too lazy to type that all out im sorry but this is one of the easiest things you'll do in chemistry and if you tried you could actually finish all 5 of these in less than a minute instead of taking like 10 min to type 10 problems out separately and making 4 diff posts

5. Geologists think that the inner and outer cores of Earth consist of
iron and nickel.
Answers
Answer:
is this true/false? if so its true.
Explanation:
Kayla is examining different soil samples and making notes about how well each sample sticks together. She has two soils that she has classified as dry, ones as wet and one as cemented. Which soil property is she examining?
a. consistency
b. density
c. structure
d. texture
Answers
a. consistency because she's making notes about how well each sample sticks together.
Answer:
A. consistency
Explanation:
I took the test
What is the molarity of 4 mol of NaOH dissolved in 2 L of water? O A. 0.5 M OB. 8 M O C. 2M D. 4 M
Answers
Answer:
concentration = mol/volume = 4/2 = 2M
What fraction of the original atoms of the radioactive sample will remain after the given number of half-lives has passed?

Answers
Answer:
1/8
Explanation:
\( \frac{1}{ {2}^{3} } = \frac{1}{8} \)
Mark brainliest please
1. The function of a buffer is to:
A) change color at the end point of a titration
B) maintain a neutral pH
C) maintain the pH of a solution
D) act as a strong acid
E) be a strong base
Answers
A buffer solution is used to maintain the pH of a solution. Buffer solution has a constant pH and it does not taking part in any reaction directly. Thus, option C is correct.
What is pH?pH of a solution is the concentration of H+ ions in the solution. It measures how acidic or basic the solution is. The solution of pH 7 is neutral and solution with pH below 7 is acidic. Basic solutions has the pH above 7.
A buffer solution is used to maintain the pH of a solution in a constant either in acidic or basic range. There are two types of buffer solutions acidic buffer and basic buffers.
An acidic buffer is made by mixing a weak acid with its salt and a basic buffer is made by mixing a weak base with its salt. A buffered solution will change its pH up on the addition of an acid or base. Hence, option C is correct.
To find more on buffer solutions, refer here:
https://brainly.com/question/13169083
#SPJ1
find the molarity of a solution with 952 grams of ammonium carbonate are dissolved to make 1750 ml of solution
Answers
The molarity of a solution with 952 grams of ammonium carbonate dissolved to make 1750 ml of solution is: 0.54 mol/L.
The molarity of a solution can be calculated by dividing the amount of solute (in this case ammonium carbonate) by the volume of the solution. In this case, 952 grams of ammonium carbonate are dissolved in 1750 mL of solution. Therefore, the molarity of the solution can be calculated as follows:
Molarity = (952 g ammonium carbonate) / (1750 mL solution) = 0.54 mol/L
To calculate the molarity, first, we need to calculate the moles of ammonium carbonate. We can do this using the molar mass of ammonium carbonate, which is 53.49 g/mol. We divide the mass of ammonium carbonate by its molar mass to get the number of moles:
(952 g ammonium carbonate) / (53.49 g/mol) = 17.77 mol
Then, we divide this number by the volume of the solution (in liters):
(17.77 mol) / (1750 mL/1000 mL/L) = 0.54 mol/L
Therefore, the molarity of the solution is 0.54 mol/L.
To know more about molarity refer here:
https://brainly.com/question/16727614#
#SPJ11
In the Lewis symbol for a fluorine atom, there are __________ paired and __________ unpaired electrons.
Answers
In the Lewis symbol for a fluorine atom, there are 4 paired and 1 unpaired electrons.
However, paired electrons simply means are 2 electrons occupying the same orbital in an atom or molecule of a substance
On the other end, unpaired electron is a single electron that occupies an orbital of an atom.
What is an atom?An atom is the smallest part of an element which can take part in a chemical reaction.
Some atoms of elements are:
MonoatomicDiatomicTriatomicPolyatomicIn conclusion, in the Lewis symbol for a fluorine atom, there are 4 paired and 1 unpaired electrons.
Learn more about paired and unpaired electrons:
https://brainly.com/question/7457518
Answer: 6, 1
Explanation:
Describe an organic molecule.
Answers
Answer: molecule of the kind normally found in living systems. Organic molecules are usually composed of carbon atoms in rings or long chains, to which are attached other atoms of such elements as hydrogen, oxygen, and nitrogen.
Explanation:
If you are given a piece of rock sugar about 2.5 cm in diameter, describe three steps you can take to dissolve it in a beaker of water in the shortest time.
Answers
Answer:
1. Crush the sugar into powder.
2. Heat the water.
3. Dissolve it by stirring continuously
Explanation:
1. Crushing the sugar into powder increases surface area. So it increases the changes of dissolving
2. Heating the water increases the capacity of water to dissolve sugar.
3. Stirring continuously increases randomness of particles so eases mixing up thus increasing dissolving tendency.
What is the acceleration acquired by an object that has a mass of 50kg and is pushed with a force of 20N.
Answers
If a 20 N force is applied to a 50 kg mass, then the acceleration acquired by the body is 0.4 m/s².
¿How to calculate the acceleration of a body?It is possible to know the acceleration of a body from Newton's second law, which states that the acceleration is defined as:
a = F/mWhere:
A = accelerationF = forceM = massTroubleshooting:We proceed to find the acceleration of the body, such that:
a = F/ma = 20N / 50kga = 0.4m/s²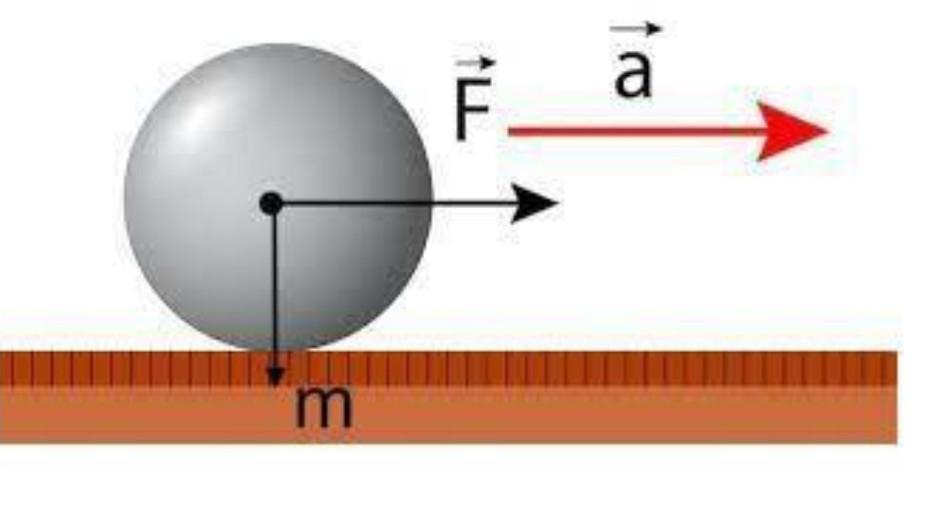
Choose the transition metal among the following which has only single ionic charge ?
A. Silver (Ag)
B. Chromium (Cr)
C. Iron (Fe)
D. Copper (Cu)
Please tell what is the answer and if possible explain me...
Answers
Answer:
Silver (Ag)
Explanation:
The electronic configuration of copper is shown below;
[Kr]4d10 5s1
We can see that there is only one 5s1 electron. Hence Ag^+ tends to display a pseudo noble gas configuration. This pseudo noble gas configuration explains why silver is prevalent in the +1 oxidation state.
The other transition metals have many stable oxidation states found in nature. Chromium is observed both in +3 and +6 oxidation states. Iron is found in +2 and +3 oxidation states and copper is mostly stable in the +2 oxidation state since the +1 oxidation state readily disproportionate.
Hence silver tends to have only a single ionic charge for reasons aptly stated above.
A vehicle is traveling at a speed of 12.5ms-1. Express this speed in light year per year
Answers
A vehicle traveling at a speed of 12.5 m/s is equivalent to 4.171 x 10⁻⁸ light years per year.
What is the speed in light year per year?To convert a speed from meters per second to light years per year, we need to use conversion factors.
1 light year = 9.461 x 10¹⁵ meters
1 year = 31,536,000 seconds
So, to convert 12.5 m/s to light years per year using the conversion factor, 1 year = 31,536,000 seconds:
12.5 m/s = 12.5 x 31,536,000 m/year
12.5 m/s = 3.9468 x 10⁸ m/year
Now, we can convert meters per year to light years per year using the conversion factor 1 light year = 9.461 x 10^15 meters:
3.9468 x 10⁸ m/year = (3.9468 x 10^8 m/year) / (9.461 x 10^15 m/light year)
3.9468 x 10⁸ m/year = 4.171 x 10⁻⁸ light years per year
Learn more about speed in light year per year at: https://brainly.com/question/803764
#SPJ1
molar mass of dinitrogen triphosphate?
Answers
The molar mass of dinitrogen triphosphide is 121 grams per mole.
What is the molar mass of dinitrogen triphosphate?The molar mass of dinitrogen triphosphide (N₂P₃) can be calculated by adding up the atomic masses of its constituent elements:
2 x atomic mass of nitrogen (N) + 3 x atomic mass of phosphorus (P)
The atomic mass of nitrogen is 14 g/mol and the atomic mass of phosphorus is 31 g/mol.
The molar mass of dinitrogen triphosphide is calculated as;
2(14 g/mol) + 3(31 g/mol)
= 121 g/mol
Learn more about molar mass here: https://brainly.com/question/21334167
#SPJ1
suppose 65.0 j of heat are added to a 105 g piece of aluminum at 22.0 ∘c.
Answers
Given the heat added (Q) and the mass (m) of the aluminum piece, we can calculate the change in temperature (ΔT) using the equation:
ΔT = Q / (c * m)
where c is the specific heat capacity of aluminum, which is approximately 0.90 J/g°C.
Plugging in the values:
ΔT = 65.0 J / (0.90 J/g°C * 105 g) = 65.0 J / 95.5 J/°C = 0.68°C
So the temperature of the aluminum piece would increase by 0.68°C to 22.68°C
Find more about Specific heat
brainly.com/question/12750526
#SPJ4
Did school teach you a lot?
Did school make your life better??
Did school make you happy?
I just wanted a other opinion
Answers
Answer: it definitely did help me a lot but sometimes it doesn’t make me happy like i will get stressed but it passes.
Explanation:
Which statement defines the heat capacity of a sample?
the temperature of a given substance
the temperature that a given sample can withstand
the quantity of heat that is required to raise the sample’s temperature by 1°C (or Kelvin)
the quantity of heat that is required to raise 1 g of the sample by 1°C (or Kelvin) at a given pressure
Answers
Answer:
b
Explanation:
Answer:
D. the quantity of heat that is required to raise 1 g of the sample by 1°C (or Kelvin) at a given pressure
Explanation:
what is the oceanic and continental crust made out of ?
Answers
Answer:
Oceanic crust is generally composed of dark-colored rocks called basalt and gabbro. It is thinner and denser than continental crust, which is made of light-colored rocks called andesite and granite. The low density of continental crust causes it to “float” high atop the viscous mantle, forming dry land.
Explanation:
Whats the different between chemical property and chemical change, and physical property and physical change?
Answers
Answer:
I think the answer is A physical property is a characteristic of a substance that can be observed or measured without changing the identity of the substance. Physical properties include color, density, hardness, and melting and boiling points. A chemical property describes the ability of a substance to undergo a specific chemical change.
Explanation:
I hope this helps.
Answer:
Physical change is the same substance, while a chemical change is a new substance.
If the statement is true, select True. If it is false, select False.
Substances that enter into a chemical reaction are called products.
True
False
Answers
Answer:
False
Explanation:
They are called products