the solubility of copper(i) chloride is 3.79 mg per 100.0 ml of solution.
Answers
The solubility of copper (I) chloride is 3.79 mg per 100.0 ml of solution. Copper (I) chloride, commonly known as cuprous chloride, is an inorganic compound containing copper and chlorine. It is a white solid that is insoluble in water but soluble in concentrated hydrochloric acid.
The solubility of copper (I) chloride is an important parameter in various fields such as electrochemistry, metallurgy, and inorganic chemistry. The solubility of copper (I) chloride depends on several factors such as temperature, pressure, and the presence of other ions.
At room temperature (25°C), the solubility of copper (I) chloride in water is very low. At this temperature, the solubility is 3.79 mg per 100.0 ml of solution. However, the solubility increases with increasing temperature. At 100°C, the solubility of copper (I) chloride in water is approximately 20 g per 100.0 ml of solution.In conclusion, the solubility of copper (I) chloride is 3.79 mg per 100.0 ml of solution at room temperature (25°C).
To know more about solubility visit:-
https://brainly.com/question/28170449
#SPJ11
Related Questions
If i add water to orange juice is that a physical change or chemical change?
Answers
Answer:
Nope, not a chemical change.
Explanation:
i hope this helps ok bye luv!!!!!!!!!!!!!!!!!!!!
Which will react with oxygen the fastest?a steel beama steel bridge an iron nailiron powder
Answers
Answer
Iron powder
Explanation
The one with the highest surface area will react with oxygen the fastest.
This is because if the surface area of a reactant is increased; more particles are exposed to the other reactant, and there is a greater chance of particles colliding, which leads to more successful collisions per second. Hence the rate of reaction increases.
The one with the highest surface area is iron powder.
Which is the balanced equation for S8 O2 → SO2? S8 O16 → 8SO2 S8 O2 → S8 O2 S8 O2 → S8O2 S8 8O2 → 8SO2.
Answers
Balanced Chemical Equation is defined as the equation in which the number of atoms on the reactants side and number of atoms on the product side is equal.
What is the balanced chemical equation?A balanced Chemical Equation is a reaction representing an equal number of atoms participating on the reactant and product sides. For example, a balanced chemical reaction between sulfur and oxygen will form the product sulfur dioxide. The balanced equation will be:\(\rm S_8 + O_2 \rightarrow 8 SO_2\)In the above equation, 8 atoms of sulfur and 2 atoms of oxygen are participating. On the product side, the balanced equation will be formed by adding 8 in front of sulfur.Thus, the balanced equation of the sulfur and oxygen will be
\(\rm S_8 + O_2 \rightarrow 8 SO_2\).
Learn more about balanced equations here:
https://brainly.com/question/7181548
Answer:
It is D
Explanation
In philosophy there is a lot of emphasis on what exists. We call this ontology, which means, the study of being. What is less often examined is what does not exist.
It is understandable that we focus on what exists, as its effects are perhaps more visible. However, gaps or non-existence can also quite clearly have an impact on us in a number of ways. After all, death, often dreaded and feared, is merely the lack of existence in this world (unless you believe in ghosts). We are affected also by living people who are not there, objects that are not in our lives, and knowledge we never grasp.
Upon further contemplation, this seems quite odd and raises many questions. How can things that do not exist have such bearing upon our lives? Does nothing have a type of existence all of its own? And how do we start our inquiry into things we can’t interact with directly because they’re not there? When one opens a box, and exclaims “There is nothing inside it!”, is that different from a real emptiness or nothingness? Why is nothingness such a hard concept for philosophy to conceptualize?
Let us delve into our proposed box, and think inside it a little. When someone opens an empty box, they do not literally find it devoid of any sort of being at all, since there is still air, light, and possibly dust present. So the box is not truly empty. Rather, the word ‘empty’ here is used in conjunction with a prior assumption. Boxes were meant to hold things, not to just exist on their own. Inside they might have a present; an old family relic; a pizza; or maybe even another box. Since boxes have this purpose of containing things ascribed to them, there is always an expectation there will be something in a box. Therefore, this situation of nothingness arises from our expectations, or from our being accustomed. The same is true of statements such as “There is no one on this chair.” But if someone said, “There is no one on this blender”, they might get some odd looks. This is because a chair is understood as something that holds people, whereas a blender most likely not.
The same effect of expectation and corresponding absence arises with death. We do not often mourn people we only might have met; but we do mourn those we have known. This pain stems from expecting a presence and having none. Even people who have not experienced the presence of someone themselves can still feel their absence due to an expectation being confounded. Children who lose one or both of their parents early in life often feel that lack of being through the influence of the culturally usual idea of a family. Just as we have cultural notions about the box or chair, there is a standard idea of a nuclear family, containing two parents, and an absence can be noted even by those who have never known their parents.
This first type of nothingness I call ‘perceptive nothingness’. This nothingness is a negation of expectation: expecting something and being denied that expectation by reality. It is constructed by the individual human mind, frequently through comparison with a socially constructed concept.
Pure nothingness, on the other hand, does not contain anything at all: no air, no light, no dust. We cannot experience it with our senses, but we can conceive it with the mind. Possibly, this sort of absolute nothing might have existed before our universe sprang into being. Or can something not arise from nothing? In which case, pure nothing can never have existed.
Chemical reactions that result in the synthesis or assembly of large molecules are referred to as: A. catabolic. B. glycolysis. C. anabolic. D. anaerobic
Answers
The chemical reactions that result in the synthesis or assembly of large molecules are referred to as anabolic.
Anabolic reactions, also known as anabolism, involve the formation of complex molecules from simpler ones. These reactions require energy and are essential for the growth and repair of cells and tissues in living organisms.. Anabolic reactions involve the synthesis or assembly of large molecules from smaller molecules, while catabolic reactions break down large molecules into smaller ones. Glycolysis is a metabolic pathway that breaks down glucose into smaller molecules, and anaerobic reactions occur without the use of oxygen.
Thus, anabolic reactions are the chemical reactions that result in the synthesis or assembly of large molecules.
To know more about anabolic reaction, click here
https://brainly.com/question/14932822
#SPJ11
what mass of magnesium bromide is formed when one grams of magnesium reacts with 5 g of bromine
Answers
Taking into account the reaction stoichiometry, a mass of 7.57 grams of MgBr₂ is formed when one grams of magnesium reacts with 5 g of bromine.
Reaction stoichiometryIn first place, the balanced reaction is:
Mg + Br₂ → MgBr₂
By reaction stoichiometry (that is, the relationship between the amount of reagents and products in a chemical reaction), the following amounts of moles of each compound participate in the reaction:
Mg: 1 moleBr₂: 1 moleMgBr₂: 1 moleThe molar mass of the compounds is:
Mg: 24.31 g/moleBr₂: 159.8 g/moleMgBr₂: 184.11 g/moleThen, by reaction stoichiometry, the following mass quantities of each compound participate in the reaction:
Mg: 1 mole ×24.31 g/mole= 24.31 gramsBr₂: 1 mole ×159.8 g/mole= 159.8 gramsMgBr₂: 1 mole ×184.11 g/mole= 184.11 gramsLimiting reagentThe limiting reagent is one that is consumed first in its entirety, determining the amount of product in the reaction. When the limiting reagent is finished, the chemical reaction will stop.
Limiting reagent in this caseTo determine the limiting reagent, it is possible to use a simple rule of three as follows: if by stoichiometry 24.31 grams of Mg reacts with 159.8 grams of Br₂, 1 grams of Mg reacts with how much mass of Br₂?
mass of Br₂= (1 grams of Mg× 159.8 grams of Br₂) ÷24.31 grams of Mg
mass of Br₂= 6.57 grams
But 6.57 grams of Br₂ are not available, 5 grams are available. Since you have less mass than you need to react with 1 grams of Mg, Br₂ will be the limiting reagent.
Mass of MgBr₂ formedConsidering the limiting reagent, the following rule of three can be applied: if by reaction stoichiometry 24.31 grams of Mg form 184.11 grams of MgBr₂, 1 grams of Mg form how much mass of MgBr₂?
mass of MgBr₂= (1 grams of Mg× 184.11 grams of MgBr₂)÷ 24.31 grams of Mg
mass of MgBr₂= 7.57 grams
Then, a mass of 7.57 grams of MgBr₂ can be produced.
Lear more about reaction stoichiometry:
brainly.com/question/12642627
#SPJ1
Use the periodic table to write the electron configuration of selenium (Se).
Answers
Answer:
1s^22s^22p^63s^23p^64s^23d^104p^4
Answer:
1s2 2s2 2p6 3s2 3p6 4s2 3d10 p4
Explanation:
PLATO correct
How many moles do you have if you have 144 L of a gas at SATP?

Answers
Answer
moles = 5.81 mol
Explanation
Given:
Volume = 144 L
AT SATP
1 mole = 24.4651 L
Solution:
1 mole = 24.4651 L
x mole = 144 L
x = 144/24.4651
x = 5.8 mol
which of the following compounds forms a racemic mixture of an optically active product when it is hydrogenated? a) i b) ii c) iii d) iv e) v
Answers
The compound that forms a racemic mixture of an optically active product when it is hydrogenated is 2-butanol.
What is optically active in a racemic mixture?
A chiral molecule is one that has two enantiomers, or nonsuperimposable mirror images of one another. An equal mixture of each of the two enantiomers of a particular chiral molecule is referred to as a racemic mixture. A racemic combination has the intriguing property of being optically inactive, which means it does not rotate plane polarized light. Actually, to be more precise, it rotates light, but it does so by an equal amount in both directions, so the net rotation is zero.To learn more about racemic mixture, follow the link https://brainly.com/question/27835707
#SPJ4
imagine you have collected data on the temperature and dissolved oxygen in a eutrophic lake over five years. you want to see if dissolved oxygen varies with temperature in this lake. what statistical test would you do ? explain why you chose this test
Answers
We can make use of the correlation approach, T test, and linear regression techniques in this situation.
The two variables in this example of a eutrophic lake are temperature and dissolved oxygen, and both of these variables change over time. Therefore, a correlation test must be performed to see whether a relationship between these two variables exists. Here, the correlation coefficient value will be given to us, allowing us to calculate the linear relationship between these two variables.
Additionally, we can use the T test to determine whether or not the correlation between these two variables is strong enough to reject the null hypothesis.
Finally, we can also utilize the linear regression method to get a more accurate relationship between those two variables.
Learn more about Hypothesis here:
https://brainly.com/question/12687557
#SPJ4
The principal mechanism for sugar dissolving in water is dissociation
Answers
The principal mechanism for sugar dissolving in water is dissociation. Sugar dissolving in water involves a process of solvation or hydration.
The polar water molecules surround the sugar molecules and break down their structure. The principal mechanism for sugar dissolving in water is not dissociation. Sugar dissolves in water due to a process called solvation, which involves the sugar molecules being surrounded by polar water molecules, breaking down the structure of the sugar.
Dissociation refers to the separation of ions or molecules in a substance that was formerly united. It occurs when an ionic compound dissolves in water, breaking down into its constituent ions. When sugar dissolves in water, it does not ionize or break down into separate ions; rather, it dissolves into individual sugar molecules.
Hence, the principal mechanism for sugar dissolving in water is solvation, not dissociation.
For more such questions on Sugar, click on:
https://brainly.com/question/4326694
#SPJ11
what is the activation energy for the isomerization of methyl isocyanide?
Answers
The activation energy for the isomerization of methyl isocyanide is 198 kJ/mol.
Isomerization is a chemical reaction in which a molecule undergoes a structural rearrangement without being broken down into smaller molecules. The activation energy is the energy that must be supplied to a chemical reaction to begin. It is a measure of the reaction's difficulty to begin.
Methyl isocyanide is a chemical compound that is a colorless gas with a powerful odor. It is a linear molecule with the chemical formula CH₃NC. This compound is of interest because of its unusual reactivity and its ability to form a stable complex with transition metals in coordination chemistry.
In the case of methyl isocyanide, the reaction pathway involves the rearrangement of a single bond from C-N to N-C. The energy required for this transformation, including the necessary breaking and formation of bonds, is known as the activation energy. It is this energy barrier that must be overcome for the reaction to proceed.
Thus, the activation energy of the isomerization of methyl isocyanide is 198 kJ/mol. This is a relatively high energy requirement, indicating that the reaction is unlikely to occur under normal conditions without additional energy input.
To know more about isocyanide refer here:
https://brainly.com/question/30826771#
#SPJ11
cyclobutane simplified structural formula please
Answers
Answer:
Cyclobutane is a cycloalkane and organic compound with the formula (CH2)4.
Explanation:
Answer:
C4H8
Explanation:
its correct on gradpoint
mark brainliest please
How many moles of O2 are used to produce 4 moles of NO?
Answers
The number of moles of O₂ used to produce 4 moles of NO is 2 moles
How do I determine the mole of O₂ used?First, shall write the balanced equation. This is given below:
N₂ + O₂ -> 2NO
From the balanced equation above,
2 moles of NO were obtained from 1 mole of O₂
With the above information, we can determine the number of moles of O₂ used to produce 4 moles of NO. This can be obtained as follow:
From the balanced equation above,
2 moles of NO were obtained from 1 mole of O₂
Therefore,
4 moles of NO will be obtain from = (4 × 1) / 2 = 2 moles of O₂
Thus, number of mole of O₂ used is 2 moles
Learn more about number of mole:
https://brainly.com/question/23350512
#SPJ1
what resource is nonrewable?
A. Pigs
B. Wheat
C. Water
D. Petroleum
Answers
Answer:
d
Explanation:
4. A researcher listed the first five ionization energies for a silicon atom in order from first to
fifth. Which of the following lists corresponds to the ionization energies for silicon?
A) 780 kJ, 13,675 kJ, 14,110 kJ, 15,650 kJ, 16, 100 kJ
B) 780 kJ, 1575 kJ, 14,110 kJ, 15,650 kJ, 16,100 kJ
C) 780 kJ, 1575 kJ, 3,220 kJ, 15,650 kJ, 16,100 kJ
D) 780 kJ, 1575 kJ, 3,220 kJ 4,350 kJ, 16, 100 kJ
E) 780 kJ, 1,575 kJ, 3,220 kJ, 4,350 kJ, 5,340 kJ
Answers
The correct list that corresponds to the ionization energy of silicon is; 780 kJ, 1575 kJ, 3,220 kJ 4,350 kJ, 16, 100 kJ
Ionization energy is the energy required to remove electrons from an atom. The number of ionization energies theoretically possible in an atom depends on the number of electrons in the atom.
However, the energy required to remove electrons from a shell in an atom increases progressively.
The second ionization energy is higher than the first ionization energy. The third ionization energy is higher than the second ionization energy and so on.
A final word, the ionization energies for electrons on the valence shell are closer to each other. However, if we go beyond the first shell into the inner shells, there is a sudden tremendous increase in ionization energy.
This jump occurs because more energy is required to remove inner electrons than is required to remove valence electrons.
We see this in silicon, the fourth ionization energy is 4,350 kJ while the fifth ionization energy of silicon is 16, 100 kJ for the reason explained above.
Learn more: https://brainly.com/question/16243729
What is the volume, measured in liters at STP, of 285 grams of the gas acetylene, C3H8?
Answers
Answer:
a
Explanation:
What is the volume, measured in liters at STP, of 285 grams of the gas acetylene, C3H8? which equals 67-4o92- witch then gives u a
Please help! Most elements' names end in the suffix -ium. This practice started around the year 1800, with many elements discovered before that having a more common name. However, some elements are more recently discovered and don't end in -ium. Do you notice any patterns for these elements? Brainliest is up for grabs!
Answers
Answer: cadmium, lanthanum, lithium, thallium, radium.
Explanation:
Think about some criteria that are important when developing a product such as toothpaste. What trade offs would you consider making to ensure that your most important criteria are met in your final design solution
Answers
Answer:
the taste and how clean the product gets your teeth, would most likely be the most important factors in making a tooth paste.
What's the purpose of chemical bonding?
Answers
Answer:
Chemical bonds hold molecules together and create temporary connections that are essential to life.
Explanation:
Chemical Bonding refers to the formation of a chemical bond between two or more atoms, molecules, or ions to give rise to a chemical compound. These chemical bonds are what keep the atoms together in the resulting compound.
Answer:
Chemical bonds hold molecules together and create temporary connections that are essential to life.
Explanation :
A chemical bond is a lasting attraction between atoms, ions or molecules that enables the formation of chemical compounds. The bond may result from the electrostatic force between oppositely charged ions as in ionic bonds or through the sharing of electrons as in covalent bonds.
Which equation is balanced?
CH4 + O2 ⟶ CO2 + H2O
Mg + 2HCl ⟶ MgCl2 + H2
Mg + P4 ⟶ Mg3P2
Answers
Answer:
A I think
Explanation:
im not sure so do with that what you will
Solid waste can be disposed by open dumping, landfill and incineration. Explain in detail the incineration system with the aid of diagram. And elaborate THREE (3) advantages and disadvantages using the incineration system to dispose the solid waste
Answers
Solid waste can be disposed by open dumping, landfill and incineration. Here, Incineration system is one of the methods of disposing solid waste. The system utilizes combustion processes to convert solid waste into ash and gases where the ash is later taken to the landfill.
Incineration is considered as a technology for ex situ thermal treatment which is based on the application of high temperature (870–1200 °C) to soil for burning harmful organic chemicals. Metals cannot be destroyed by using this technique. The efficiency of a properly operated incinerator is very high, especially for PCBs and dioxins. Incineration differs from the thermal desorption system in that incineration needs higher temperatures to chemically oxidize or decompose the contaminants, whereas the second method only volatilizes them.
The Advantages of incineration system of solid waste disposal includes:
1. Reduction in the volume of waste ,Incineration reduces the volume of waste to 10% to 20% of its original size.
2. Generation of electricity Incineration plants generate electricity by harnessing the heat energy that is generated by burning solid waste.
The Disadvantages of incineration system of solid waste disposal includes:
1. Production of toxic fumes Incineration of certain materials can lead to the production of toxic fumes such as dioxins.
2. The installation of incineration plants is quite expensive. The plants require regular maintenance to remain in good working condition. For this reason municipalities and cities that have to bear the cost.
To know more about solid waste here
https://brainly.com/question/16044669
#SPJ4
NaOH + HCI → NaCl + H20
*double replacement
*single replacement
*synthesis
*combustion
Answers
Answer: a double replacement
Explanation:
which particle is not used in calculating the atomic mass?
Answers
The smallest and least massive atomic particle is the electron, which is negatively charged.Due to the electron's extremely small mass, it is not counted inside the element's atomic number.
What particle does not add to mass?Although they are very small and possess a mass of 1/1850 of that of a protons or neutron, electrons carry a negative charge.Since they are so tiny, they do not, in reality, add to the weight of the atom.
What three particles make up an atom?These particles are frequently referred to it as subatomic particles since they are the building blocks of atoms.There are protons, neutrons, and electrons, three types of subatomic particles.Protons and electrons, two of a subatomic particles, each have an electrical charge of one or the other.
To know more about electron visit:
https://brainly.com/question/1255220
#SPJ4
when a compound donates (loses) electrons, that compound becomes blank. such a compound is often referred to as an electron donor.target 1 of 6 2. when a compound accepts (gains) electrons, that compound becomes blank. such a compound is often referred to as an electron acceptor.target 2 of 6 3. in glycolysis, the carbon-containing compound that functions as the electron donor is blank.target 3 of 6 4. once the electron donor in glycolysis gives up its electrons, it is oxidized to a compound called blank.target 4 of 6 5. blank is the compound that functions as the electron acceptor in glycolysis.target 5 of 6 6. the reduced form of the electron acceptor in glycolysis isblank.target 6 of 6
Answers
when a compound donates (loses) electrons, that compound becomes Oxidized. such a compound is often referred to as an electron donor.
when a compound accepts (gains) electrons, that compound becomes Reduced. such a compound is often referred to as an electron acceptor.
in glycolysis, the carbon-containing compound that functions as the electron donor is Glucose.
once the electron donor in glycolysis gives up its electrons, it is oxidized to a compound called Pyruvate
5. NAD⁺ is the compound that functions as the electron acceptor in glycolysis.
6. the reduced form of the electron acceptor in glycolysis is NADH
A substance is said to be oxidized when it loses electrons, loses hydrogen, or receives oxygen.
However, the opposite happens with reduction. In other words, when a substance gains electrons, gains hydrogen, or loses oxygen, it is said to be reduced.
A substance that itself is reduced to oxidize another substance is called an oxidizing agent. Redox reactions occur simultaneously. That is, when one substance is oxidized, only another substance is reduced.
During glycolysis, the six-carbon compound glucose (C6H12O6) loses electrons and is finally converted to a three-carbon compound known as pyruvate (CH3COCOOH). In this process, an oxidant called NAD+ receives electrons and is itself reduced to NADH.
The chemical formula for glucose is C6H12O6, while the chemical formula for pyruvate is CH3COCOOH. Looking at their chemical formulas, it is easy to see that glucose has lost hydrogen compared to pyruvate. That means that glucose has been oxidized. Glucose has 12 hydrogens for 6 carbons, but pyruvate has only 4 hydrogens for 3 carbons. This means that glucose was oxidized in the process because the molecule produced, pyruvate, has relatively few hydrogens.
learn more about glucose oxidation at https://brainly.com/question/24542799
#SPJ4
I need the answer asap

Answers
Answer:
The energy will be added
Explanation:
PLEASE HELP 10 POINTS
Which of the following is a
reasonable ground-state electron configuration?
1s21p5
1s22s22p8
1s22s22d4
1s22s22p6
Answers
The reasonable ground - state electron configuration is 1s² 2s²2p⁶
1) Electron configuration is defined as the arrangement of electron in the atomic orbtials.
2)The ground state electron configuration is lowest energy level and have most stable arrangement.
3) An excited state configuration is a higher energy level.
4) According to Aufbau principle ., electrons will occupy the orbitals of lower energies first then occupying higher energy orbitals.
5) Energy of orbitals is calculated by the sum of principle quantum number and azimuthal quantum numbers.
6) The rule of electron filling in the orbital is from lower energy level to higher energy level.
7) According to this rule electrons are filled in the order of 1s 2s 2p 3s 3p 4s 3d 4p 5s ........and so on.
8) According to pauli principle , maximum two electrons, having opposite spin can fit in orbital.
9) According to Hund's rule , orbital in given sub-shell is singly occupied by electrons before filling second electron in an orbital.
10) The ground state configuration is the arrangement of electron around the nucleus of an atom with lower energy levels.
Hence,The reasonable ground-state electron configuration is 1s² 2s² 2p⁶
To learn more about electronic configuration here
https://brainly.com/question/14283892
#SPJ1
Which statement is true of any reversible reaction?
A. It can proceed in either direction
B. It can occur only between gases
C. It has only one product
D. It has more than one reactant

Answers
Answer:
A. It can proceed in either direction
Explanation:
A P E X
Reactions can be reversible and irreversible. The reversible reactions can proceed in either direction. Thus, option A is correct.
What are reversible reactions?Reversible reactions can convert the reactants and products into each other and hence, move in both the left and right directions. These types of reactions never get completed and attain equilibrium.
The reactants react to produce the products, and the products re-react to form the reactants of the reaction mixture. The melting of the wax can be a reversible reaction.
Therefore, option A. reversible reactions can occur in both directions is correct.
Learn more about the reversible reaction here:
https://brainly.com/question/23711430
#SPJ2
16. For the reaction below, A) calculate the total bond energy, using the equation: Reactant energy - product energy = total energy.
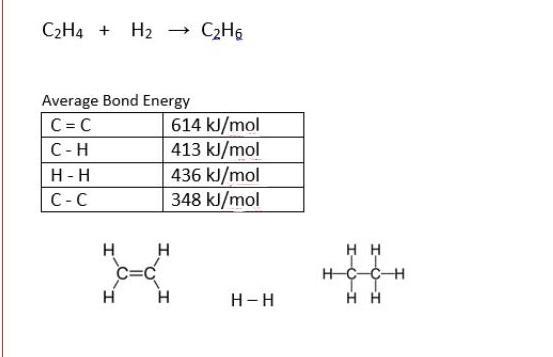
Answers
Answer:
C=C divided by the co effiecient 11 would make the denominator of the photosynthesis equal 11 over 5 making this problem a polywhirl which wouldnt matter in the sense of the quake in energy change so thus making the answer 728 kJ/mol
Explanation: back in my day
How does an indicator show the difference between an acid and an alkali?
Answers
Explanation:
When a base dissolves in water it is an alkali and makes an alkaline solution.
...
Litmus.
Red Litmus Blue Litmus
Acidic solution Stays red Turns red
Neutral solution Stays red Stays blue
Alkaline solution Turns blue Stays blue
What property can be used to determine if a sample is a pure substance or a mixture?
Select one:
color
temperature
melting point
mass
Answers
Answer:
Melting point
Explanation:
Pure substances have sharp melting and boiling points while impurities lower the melting point and raise the boiling point