The pattern produced by the variable rates at which components of a solution travel is known as the.
Answers
The pattern produced by the variable rates at which components of a solution travel is known as the Chromatogram.
Chromatography is a laboratory technique used to separate and purify mixtures. The sample is dissolved in a mobile phase, which is then passed through a stationary phase. The various components of the sample interact with the stationary phase to varying degrees, causing them to travel at different rates through the stationary phase. The time it takes for a component to pass through the stationary phase is referred to as its retention time.
The chromatogram is a visual representation of the separation. The peaks in the chromatogram represent individual components of the mixture. The height of each peak is proportional to the amount of that component in the mixture. By measuring the area under each peak, the relative amounts of each component can be determined. Chromatograms are used in a wide range of fields, including chemistry, biology, and forensics.
Learn more about chromatogram here:
https://brainly.com/question/10874556
#SPJ11
Related Questions
KINDLY PARAPHRASE THE FOLLOWING PARAGRAPHS:
-------------------------------------------------------------------------------------------------------------------------
Growth in Distribution Spaces
An essential part of the e-commerce business is its supply chain. Figuring out the logistics for packaging and shipping goods to customers includes warehousing, and that’s where commercial real estate comes into play. As e-commerce has grown, we have seen significant growth in the leasing and sale of distribution centers and warehouse spaces.
E-commerce giants look for spaces near large cities like Houston while still having enough space for large buildings. There is a lot of potential and growth in the Houston suburbs such as Katy, Brookshire & Waller. We are seeing more distribution centers popping up in these areas.
Smaller Retail Spaces
As retail has shifted to online, we have seen businesses struggling to keep physical spaces open over the past few years. While e-commerce is booming, some brick-and-mortar spaces are having to close or downsize.
There are certain markets, like groceries, that will always require a physical location, but there is a trend for smaller retail spaces across the market. Smaller spaces mean less inventory in-store, and this consequently encourages a combination of online and in-store shopping. Hybrid shopping especially increased in popularity during the Covid-19 lockdown.
Merging online shopping with curbside or in-store pick-up offered that element of convenience and a safe way to shop during the pandemic, and even as restrictions ease, people will still seek the ease of this approach. However, even though convenience is what mainly drives e-commerce, we don’t expect to see in-store experiences disappear altogether.
Increased Technology in Retail
Since many prefer shopping online, working to translate the benefits of technology to physical spaces has been important in keeping up with trends. Integrating technology into retail spaces will be essential for future leasing and selling opportunities in the market. Implementing tools such as apps can create unique and convenient shopping experiences and can help businesses gather data that is essential for tracking traffic and learning more about the customer.
These tools can also help drive customers to the retail location with special offers or in-store pickup options. Large lifestyle shopping centers have shown to be among the most proactive in blending technology with consumer experiences.
Overall, e-commerce has a major impact on the commercial real estate business, from the industrial real estate benefit from its growth to seeing space buying and leasing becoming a smaller part of retail operations. In 2020 alone, e-commerce accounted for 14 percent of all sales, but it is inevitable that e-commerce will continue to grow as it has for the last decade. Commercial real estate is a reflection of society and its habits and we will continue to see it mirrored as changes in technology and retail emerge.
---------------------------------------------------------------------------------
Answers
The impact of e-commerce on commercial real estate is significant. E-commerce sales have grown steadily, accounting for a considerable portion of overall sales.
The growth of e-commerce has fueled the demand for distribution spaces, specifically distribution centers and warehouses, which play a crucial role in the supply chain and logistics of packaging and shipping goods to customers. These spaces are sought after by e-commerce giants, who prefer locations near large cities while still providing ample room for large buildings. Suburban areas, such as Katy, Brookshire, and Waller near Houston, are experiencing significant growth in the establishment of distribution centers.
On the other hand, the rise of online shopping has posed challenges for brick-and-mortar retailers. Many physical retail spaces have struggled to remain open or have had to downsize. As a result, there is a trend towards smaller retail spaces, which require less inventory in-store. This trend encourages a combination of online and in-store shopping, known as hybrid shopping. The Covid-19 pandemic further accelerated this trend as consumers sought the convenience and safety of online shopping with options like curbside or in-store pick-up. Even as restrictions ease, this approach is expected to remain popular.
To adapt to the changing retail landscape, integrating technology into physical retail spaces has become crucial. Technology tools, such as mobile apps, can enhance the shopping experience, offer special promotions, and provide valuable data on customer behavior. Retailers, especially large lifestyle shopping centers, have been proactive in blending technology with consumer experiences to stay relevant and attract customers.
Learn more about Covid-19 pandemic here:
https://brainly.com/question/30975256
#SPJ11
The processes in distillation involve *
Answers
Explanation:
distillation, process involving the conversion of a liquid into vapour that is subsequently condensed back to liquid form. ... This basic operation requires the use of a still or retort in which a liquid is heated, a condenser to cool the vapour, and a receiver to collect the distillate.
Plz follow me and Mark me as brainlest please
A 48.6-ml sample of gas in a cylinder is warmed from 19°C to 81°C. What is its volume at the final temperature?
Answers
Answer:
V2=58.9
Explanation:
V1=48.6
T2=81°C=354K
T1=19°C=292K
V2=X
V2=V1×T2÷T1
X=48.6×354÷292
X=17204.4÷292
X=58.9
V2=58.9
Perform the followingmathematical operation, andreport the answer to thecorrect number of significantfigures.0.97584 = 5.68 = [ ?]=Enter
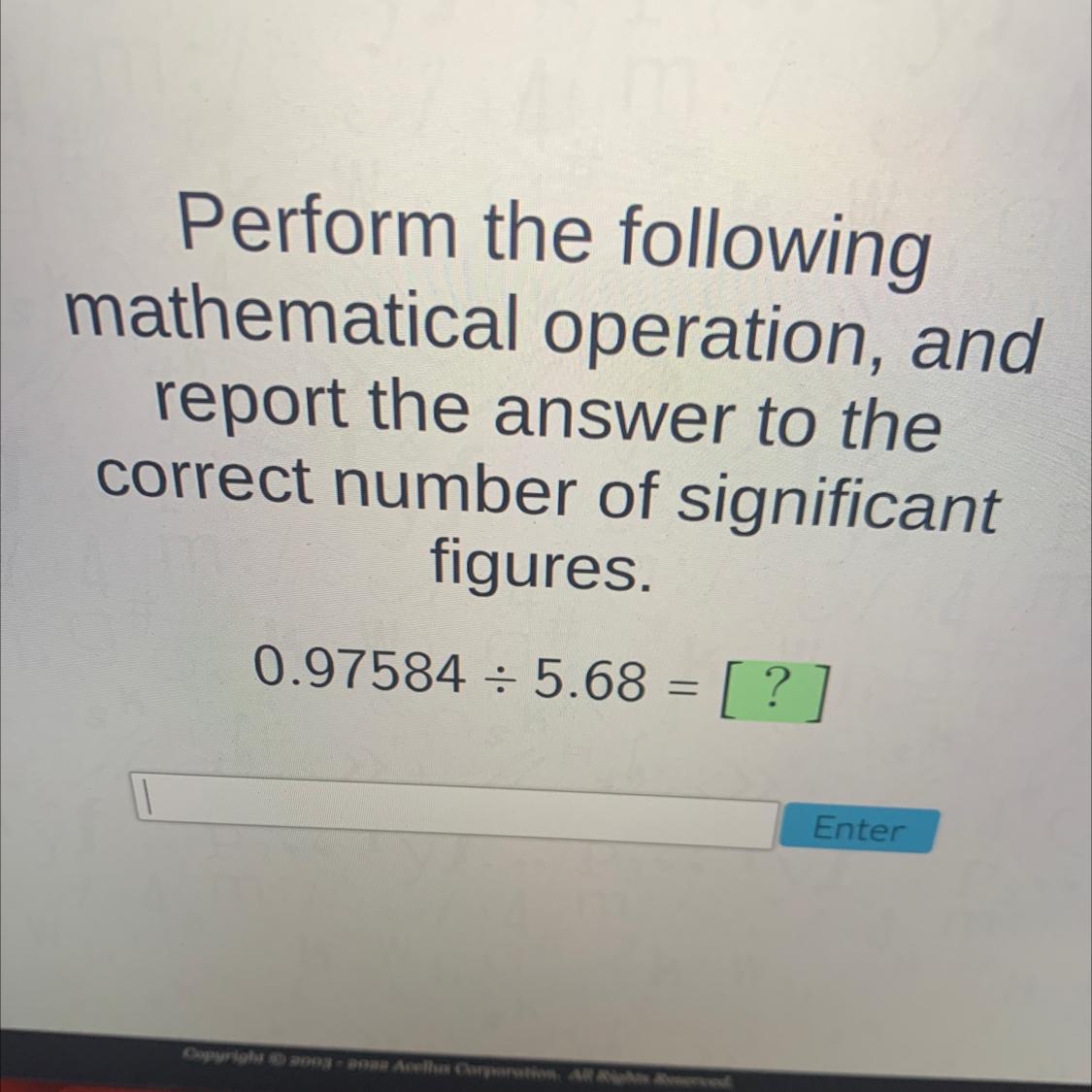
Answers
When we do the respective division operation we get the following result 0.171802.
When we do the respective division operation we get the following result 0.171802. But we must adjust the significant figures taking into account the number that has less significant figures, which is 5.68.
5.68 has three significant figures. So we will adjust the significant figures of the result to three.
0.97584 / 5.8 = 0.1718 = 0.172
explain the process that creates chemical elements lighter than iron, including evidence
Answers
The process that creates chemical elements lighter than iron is known as nuclear fusion and the evidence is the fusion of carbon and helium nuclei which forms an oxygen nucleus, and so on.
What is Nuclear fusion?This is referred to as the type of reaction which is characterized by two or more atomic nuclei being combined to form one or more different atomic nuclei and subatomic particles.
It is also responsible for the creation of chemical elements lighter than iron through successive reactions and a common example is the fusion of carbon and helium nuclei to form an oxygen nucleus thereby making it the correct choice.
Read more about Nuclear fusion here https://brainly.com/question/982293
#SPJ1
I have 5 questions that need help to answer 1) What are covalent bonds and how do they form ? 2) How do you know which elements and how many of each are in a compound ?
3) How can you find how many valence electrons an atom has ?
4) How many valence electrons do atoms need to be happy? Which elements are the exceptions?
Answers
Answer:
covalent bond- a chemical bond that is the sharing of electron pairs between atoms
Complete the following reaction.
14/7 N + 1/0 n —>
blank/blank C+1/1 H

Answers
Answer:
14/6
Explanation:
U 2 can help me by marking as brainliest........
There can be emissions of radiations like gamma radiation. There can be emission of particles too like alpha particle. Therefore, the complete reaction is ¹⁴N₇ + ¹n₀ \(\rightarrow\) ¹⁴ C₆ + ¹H₁.
What is nuclear decay?Nuclear decay is process in which the radioactive element releases particles or radiations. Alpha particles is ⁴₂He. Alpha particle is nothing but helium particle. There are so many types of nuclear decay. The kinetic for decay is of first order kinetics.
The balanced equation is the one in which the atomic number and mass number of element is always conserved. So, for the given decay, balanced reaction can be written as below
¹⁴N₇ + ¹n₀ \(\rightarrow\) ¹⁴ C₆ + ¹H₁
Generation of carbon takes place from nitrogen
Therefore, the complete reaction is ¹⁴N₇ + ¹n₀ \(\rightarrow\) ¹⁴ C₆ + ¹H₁.
To know more about nuclear decay, here:
https://brainly.com/question/21114779
#SPJ2
A clear colorless liquid, Barium Chloride (BaCl), is added
to a clear blue solution of copper sulfate (CuSO4). The
solution tums pale blue and cloudy. After a while, the
cloudy substance all sinks to the bottom of the test tube.
Physical or chemical change and explain.
Answers
Answer:true
Explanation:
This statement is correct
how did rutherford interpret the fact that most of the ????α particles seemed to pass right through the foil?
Answers
Ernest Rutherford gave the atomic model based on the centrally located mass of the atom. The α particles passed through the foil as an atom has empty spaces.
What is Rutherford's atomic theory?Rutherford gave the atomic model by bombarding the gold foil with an alpha particle from radioactive material. It gave the atomic structure constituting the sub-atomic particles.
Most of the alpha particles passed straight through the foil which concluded that most of the space in the atom was empty and the major mass was accumulated at the center.
Therefore, the α particles passed straight as most of the atom has empty spaces.
Learn more about Rutherford's atomic theory, here:
https://brainly.com/question/28427666
#SPJ4
A pencil has density of 0.875 g/cm^3. It has a volume of 4.0 cm^3. What is the mass?
Answers
Answer:
3.5g
Explanation:
\(Density=\frac{mass}{volume}\\ Mass= Density X Volume\\Mass = 0.875 X 4.0\\Therefore, Mass = 3.5 g\)
Answer:
3.703703703703
Explanation:
CNH Radiology centre provides services for X-Ray procedures on per patient basis, $100 per patient:
According to the forcast, its patient number in 2018 will be 2000 for Q1, 6000 for Q2, 8000 for Q3, 4000 for
04. Generally, around 70% of the X-ray procedure revenue will be collected in current quarter and rest 30%
will be collected in next quarter. At the last quarter of 2017, outstanding Accounts Receivable shows
S90,000 on its Dec31, 2017 balance sheet.
Four X-Ray films will be used up for each of patient. Desired ending inventory is 10% of next quarter's need
The 2017 04 ending inventory are 400 sheets and the 2018 Q4 desired ending inventory are 1000 sheets.
The Average purchase cost per sheet will be $4.74 for Q1, $3.919 for Q2. $3.385 for Q3 and $3.624 for Q4
accordingly.
Generally, it takes 1.5 hour for the X-ray technician to complete X-Ray procedure for each patient.
The hourly pay rate for technician is average $20 per labour hour.
CNH's overhead costs can be generally divided into two categories---variable part and fixed part. Please
note its predetermined overhead rate for the year will be $5 per labor hour. According to the data,
Its fixed part of the overhead costs are stable every year-…-$242,400 including $60,000 amortization.
Radiology centre's cash balance at the end of 2017 was $42,500. Its office building administrative cost will
be Q1 $93,000; Q2 $130,900, Q3 $184,750, Q4 $129,150 respectively. According to the management, Radiology
centre will have equipment purchase in 2018, Q1 $89,400, Q2 $66,204, Q3 $1,602, Q4 $29,393.
Please make a cash budget to see if the Radiology centre has enough cash-in flow to cover the expenditures.
If Radio centre fall into deficiency, it will have to finance from the bank. The borrowing interest rate is 10%.
If there is a cash excess during the budget period, funds borrowed in previous periods can be repaid. Please
Note that Radiology centre must maintain at least keep $40,000 cash balance each quarter just in case 9$,
the emergency.
revenue budget
Answers
Revenue Budget: Projected revenue for CNH Radiology Centre in 2018:
Q1: $200,000 (2000 patients * $100 per patient)
Q2: $600,000 (6000 patients * $100 per patient)
Q3: $700,000 [($800,000 * 70%) + ($400,000 * 30%)]
Q4: $480,000 [($400,000 * 70%) + ($0 * 30%)]
Based on the forecasted patient numbers and the revenue per patient, the revenue budget for CNH Radiology Centre in 2018 is as follows. In Q1, with 2000 patients, the revenue is projected to be $200,000. In Q2, with 6000 patients, the revenue is expected to reach $600,000. For Q3, the revenue is calculated by considering 70% of the expected revenue from Q3 patients and 30% from Q4 patients. Thus, the total revenue for Q3 is projected to be $700,000. Similarly, for Q4, the revenue is calculated using 70% of the expected revenue from Q4 patients and 30% of the revenue from Q1 patients, as there are no forecasted patients for Q4. Therefore, the total revenue for Q4 is expected to be $480,000.
Learn more about CNH Radiology here:
https://brainly.com/question/30388738
#SPJ11
A gas has a volume of 1400 milliliters at a temperature of 20 K and a pressure of 760 mm Hg. What will be the
volume when the temperature is changed to 40 K and the pressure is changed to 380 mm Hg?
A. 350 mL
B. 750 mL
c. 1400 ml
D. 5600 mL
Answers
Answer: 1400 mL
Explanation:
The change in volume with change in pressure and change in temperature can be calculated using combination of gas laws. Here, the change in volume is 5600 ml.
What is combined gas laws?Boyle's law, Charles's law and Gay- Lussacs law all relating the temperature, pressure and volume of an ideal gas. The relation between these three parameters from the combination of these laws at conditions is written as:
P1 V1/T1 = P2 V2/ T2.
From this, the change in volume V2 can be calculated.
Given , P1 = 760 mmHg
T1 = 20 k and V1 = 1400 mL.
T2 = 40 k and P2 = 380 mmHg.
The new volume V2 = P1 V1 T2 / T1 P2.
= (760 mmHg × 1400 ml × 40K) / (20 K × 380 mmHg)
= 5600 mL.
Therefore, the change in volume of the gas is 5600 ml.
To find more on combined gas laws, refer here:
https://brainly.com/question/28257995
#SPJ2
An alkene X undergoes ozonolysis and gives two compounds Y and Z of molecular formula CaHO. Y and Z are functional isomers of each other i. Write the two-steps chemical equation for the conversion of X into Y and Z. [2] ii. Write the structural formula of Y and Z. Why are they called functional isomers? [3] iii. What happens when hydrogen gas in the presence of nickel catalyst is passed over X? [1] iv. What is the application of ozonolysis in the organic reaction mechanism? [1] How can you prove chemically the compound X is unsaturated?
Answers
i. The given compound X is 2-methyl pent-2-ene. When it is reacted with ozone it forms an ozonide in the first step. In the second step the reduce to forms acetone and propanal.
ii. The structural formula of Y is \(CH_3-CO-CH_3\) and Z is \(CH_3-CH_2-CHO\).
iii. Alkenes, upon catalytic hydrogenation, form alkanes. This will occur in the presence of Nickel as the catalyst.
iv. The process of ozonolysis is useful in the field of pharmaceutics.
v. The test of unsaturation can be performed by passing a compound through Bromine water.
What is ozonolysis?Ozonolysis is a reaction used in organic chemistry to determine the position of a carbon-carbon double bond in unsaturated compounds.
i. The given alkene X, that is subject to ozonolysis would be 2-methyl-2-pentene. Upon exposure to ozone, an ozonide is initially formed, after which it is broken down into 2 products - acetone and propanal, both with the molecular formula C₃H₆O.
The given compound X is 2-methyl pent-2-ene. When it is reacted with ozone it forms an ozonide in the first step. In the second step the reduce to forms acetone and propanal.
ii. The formulas of Y is \(CH_3-CO-CH_3\) and Z is \(CH_3-CH_2-CHO\). They are functional isomers as they have the same molecular formula but different functional groups - ketone and aldehyde.
iii. When alkenes undergo catalytic hydrogenation, they form alkanes, X will form 2 methyl petane on reaction with hydrogen gas in presence of Ni.
iv. The ozonolysis is used for the industrial-scale synthesis of pharmaceuticals.
v. The unsaturation of compound X can be proved by the bromine water test. As on reaction with it, the brown colour of bromine water becomes colourless due to the formation of dibromo alkane.
Learn more about the ozonolysis here:
https://brainly.com/question/14356308
#SPJ1

If a balloon in a small airplane cargo container
that has an air pressure of 0.800 atm and a
volume of 1.00 L at 15
"C, what would the volume
of the balloon shrink to when the plane lands and
the balloon is exposed to 25 °C and 1.00 atm?
Answers
When the plane lands and the balloon is exposed to a temperature of 25 °C and a pressure of 1.00 atm, the volume of the balloon will shrink to approximately 0.925 L.
When the balloon in a small airplane cargo container, initially at a volume of 1.00 L, is exposed to a different temperature and pressure during landing, its volume will change. To calculate the final volume of the balloon, we can use the combined gas law equation:
(P₁V₁) / (T₁) = (P₂V₂) / (T₂)
Where P₁, V₁, and T₁ represent the initial pressure, volume, and temperature of the balloon, and P₂, V₂, and T₂ represent the final pressure, volume, and temperature.
Given that the initial conditions are P₁ = 0.800 atm, V₁ = 1.00 L, and T₁ = 15 °C = 288 K, and the final conditions are P₂ = 1.00 atm, T₂ = 25 °C = 298 K, we can rearrange the equation to solve for V₂, the final volume of the balloon.
Using the given values and solving for V₂, we find that the final volume of the balloon is approximately 0.925 L.
Therefore, when the plane lands and the balloon is exposed to a temperature of 25 °C and a pressure of 1.00 atm, the volume of the balloon will shrink to approximately 0.925 L.
Know more about Gas Law here:
https://brainly.com/question/27009857
#SPJ11
How many grams are in 2.190mol Mg2(SO4)3?
Answers
Mass MgSO₄ = 262.8 g
Further explanationGiven
2.190 mol MgSO₄
Required
mass
Solution
The molecular formula should be MgSO₄
So MW MgSO₄ :
= Ar Mg + Ar S + 4. Ar O
= 24 + 32 + 4. 16
= 120 g/mol
Mass MgSO₄ :
\(\tt mass=mol\times MW\\\\mass=2.19\times 120\\\\mass=262.8~g\)
What is the molar concentration a a 12 % sodium chloride solution (MW 58.5)
Answers
The molar concentration of a 12% sodium chloride solution is approximately 2.05 M.
To determine the molar concentration of a 12% sodium chloride solution, we need to convert the given percentage concentration into molarity.
First, we need to understand that the percentage concentration refers to the mass of the solute (sodium chloride) relative to the total mass of the solution.
In this case, a 12% sodium chloride solution means that there are 12 grams of sodium chloride in 100 grams of the solution.
To convert this into molar concentration, we need to consider the molar mass of sodium chloride, which is 58.5 g/mol.
We can start by calculating the number of moles of sodium chloride in 12 grams:
Moles of sodium chloride = mass of sodium chloride / molar mass of sodium chloride
Moles of sodium chloride = 12 g / 58.5 g/mol = 0.205 moles
Next, we calculate the volume of the solution in liters using the density of the solution. Since the density is not provided, we assume a density of 1 g/mL for simplicity:
Volume of solution = mass of solution / density
Volume of solution = 100 g / 1 g/mL = 100 mL = 0.1 L
Finally, we calculate the molar concentration (Molarity) by dividing the number of moles by the volume in liters:
Molar concentration = moles of solute / volume of solution
Molar concentration = 0.205 moles / 0.1 L = 2.05 M
Therefore, the molar concentration of a 12% sodium chloride solution is approximately 2.05 M.
To learn more about molarity click here: brainly.com/question/31545539
#SPJ11
Why do we emphasize the valence electrons in an atom when discussing atomic properties?
Answers
We emphasize the valence electrons in an atom when discussing atomic properties, as they are most accessible to other atoms.
What are valence electrons?The valence electrons are the electrons that are present in the outer shell of the atoms. These electrons are free for making bonds with the electrons of other atoms.
Electrons are joined with other electrons to make a compound. These electrons are shared by or gained by other atoms.
Thus, when discussing atomic qualities, we focus on the valence electrons in an atom since they are the most receptive to other atoms.
To learn more about valence electrons, refer to the link:
https://brainly.com/question/13993867
#SPJ4
If you help ill give brainlest i need this done almost asap please
If an atom is highly reactive, a metal, and has one valence electron, what group/family does it belong to? (Name and number)
Answers
Answer:The answer is group 1 and alkali metals
Explanation:The elements in group one are alkali metals and have 1 valance electron
to convert a concentration unit based on mass to one based on volume, the of the solution will be required.
Answers
Answer:
To convert a concentration unit based on mass to one based on volume, we need to know the density of the solution. The density gives us the mass of the solution per unit volume. Once we know the density, we can use it to convert between mass-based and volume-based concentration units.
For example, let's say we have a solution that is 20% (w/w) glucose. This means that 20 grams of glucose are dissolved in 100 grams of solution. To convert this to a concentration unit based on volume, we need to know the density of the solution. Let's assume that the density is 1.2 g/mL.
First, we can calculate the mass of the solution:
100 g solution = 20 g glucose + 80 g solvent
Next, we can calculate the volume of the solution:
100 g solution / 1.2 g/mL = 83.33 mL solution
Now we can calculate the concentration of glucose in the solution based on volume:
20 g glucose / 83.33 mL solution = 0.24 g/mL or 24% (w/v) glucose
So the concentration of the solution based on volume is 24% (w/v) glucose.
Using the activity series (Table 4.5), write balanced ical equations for the following reactions. If no occurs, simply write NR. (a) Iron metal is added solution of copper(II) nitrate; (b) zine metal is added a solution of magnesium sulfate, () hydrobromic ad added to tin metal; (d) hydrogen gas is bubbled tho an aqueous solution of nickelh) chloride; le) aluminun metal is added to a solution of cobalt(I1) sulfate
Answers
a) Fe + Cu(NO3)2 -> Fe(NO3)2 + Cu;
b) Zn + MgSO4 -> ZnSO4 + Mg;
c) 2HBr + Sn -> SnBr2 + H2;
d) H2 + NiCl2 -> 2HCl + Ni;
e) 2Al + CoSO4 -> Al2(SO4)3 + Co. These are the balanced chemical reactions.
(a) Fe(s) + Cu(NO3)2(aq) → Cu(s) + Fe(NO3)2(aq)
(b) Zn(s) + MgSO4(aq) → NR
(c) 2HBr(aq) + Sn(s) → SnBr2(aq) + H2(g)
(d) H2(g) + NiCl2(aq) → Ni(s) + 2HCl(aq)
(e) 2Al(s) + 3CoSO4(aq) → 3Co(s) + Al2(SO4)3(aq)
In response (a), iron is more receptive than copper and in this way uproots copper from the copper(II) nitrate arrangement.
In response (b), zinc is less receptive than magnesium, so no response happens.
In response (c), hydrobromic corrosive is more receptive than tin and in this way uproots tin from the strong state, shaping tin(II) bromide and hydrogen gas.
In response (d), hydrogen gas is less receptive than nickel and hence no response happens.
In response (e), aluminum is more receptive than cobalt and hence dislodges cobalt from the cobalt(II) sulfate arrangement.
To learn more about the balanced chemical reaction, refer:
https://brainly.com/question/5848932
#SPJ4
The complete question is:
Using the activity series(Table 4.5), write balanced chemical equations for the following reactions. If no reaction occurs, simply write NR. (a) Iron metal is added to a solution of copper(II) nitrate; (b) zinc metal is added to a solution of magnesium sulfate; (c) hydrobromic acid is added to tin metal; (d) hydrogen gas is bubbled through an aqueous solution of nickel(II) chloride; (e) aluminum metal is added to a solution of cobalt(II) sulfate.
A solution of cobalt(II) chloride is analyzed, and the concentration of cobalt(II) ions
is found to be 0.27 mol/L. What is the expected concentration of chloride ions?
Answers
The expected concentration of chloride ions is 0.54 mol/L
What is molarity?
Molarity of a given solution is defined as total number of moles of solute per litre of solutionExample-One mole of sodium chloride weighs 58.44 grams , if you dissolve 58.44 grams of NaCl in one liter of water, we have one molar solution, abbreviated as 1M.Most commonly used unit for molarity is number of moles per liter, having the unit symbol mol/L or mol⋅dm⁻³ in SI unitChemical formula of Cobalt Chloride is CoCl₂
[Co⁺² ] = 0.27 mol/L (given)
CoCl₂ in aqueous solution ionizes as follows:
CoCl₂ (aq) → Co⁺² (aq) + 2Cl⁻ (aq)
On ionizing CoCl₂ , 2 moles of chlorine is produced. Thus concentration of chlorine will be equal to :
[Cl⁻] = 2 × [ Co⁺² ]
= 2 × 0.27
= 0.54 mol/L
Learn more about Molarity at https://brainly.com/question/14469428
#SPJ13
Sarah measures out 151 grams of SO2. How many moles is this? Express your answer to three significant figures.
Answers
Answer:
\(\boxed {\boxed {\sf 2.36 \ mol \ SO_2}}\)
Explanation:
We are asked to convert grams to moles. We will use the molar mass and dimensional analysis to perform this conversion.
1. Molar MassThe molar mass is the mass of 1 mole of a substance. These values are found on the Periodic Table because they are equivalent to the atomic masses, but the units are grams per mole instead.
We are given a mass of sulfur dioxide (SO₂). Look up the molar masses of the individual elements.
Sulfur (S): 32.07 g/mol Oxygen (O): 15.999 g/molNotice that the formula of the compound contains a subscript. The subscript after O means there are 2 moles of oxygen in 1 mole of sulfur dioxide. We must multiply oxygen's molar mass before adding sulfur's.
O₂: 15.999 * 2 = 31.998 g/mol SO₂= 32.07 + 31.998 = 64.068 g/mol2. Convert Grams to Moles
Now we will use dimensional analysis to convert grams to moles. From the molar mass, we know there are 64.068 grams of sulfur dioxide per mole, so we can set up a ratio.
\(\frac {64.068 \ g \ SO_2} {1 \ mol \ SO_2}\)
We are converting 151 grams to moles, so we multiply by this value.
\(151 \ g \ SO_2 *\frac {64.068 \ g \ SO_2} {1 \ mol \ SO_2}\)
Flip the ratio so the units of grams of sulfur dioxide cancel.
\(151 \ g \ SO_2 *\frac {1 \ mol \ SO_2}{64.068 \ g \ SO_2}\)
\(151 *\frac {1 \ mol \ SO_2}{64.068 }\)
\(\frac {151}{64.068 } \ mol \ SO_2\)
\(2.356870825 \ mol \ SO_2\)
3. RoundThe original measurement of grams (151) has 3 significant figures, so our answer must have the same. For the number we calculated, that is the hundredth place. The 6 in the thousandth place tells us to round the 5 in the hundredth up to a 6.
\(2.36 \ mol \ SO_2\)
151 grams of sulfur dioxide is approximately 2.36 moles of sulfur dioxide.
How are sodium (Na) and potassium (K) similar?
O A. They are hard metals.
B. They are highly reactive.
C. They are soft nonmetals.
D. They rarely react.
Answers
write bohrs atomic theory postulate
Answers
Answer:
Hey mate....
Explanation:
This is ur answer....
1) All matter is made of atoms. Atoms are indivisible and indestructible. 3) Compounds are formed by a combination of two or more different kinds of atoms. 4) A chemical reaction is a rearrangement of atoms.
Hope it helps you,
Mark me brainliest plz....
Follow me!
Which of the following chemical equations depicts a balanced chemical reaction?A. H2+O2−>H2OB. 2H2+O2−>2H2OC. 2H2+2O2−>H2OD. 2H2+2O2−>2H2O

Answers
The chemical equation that depicts a balanced chemical reaction is the one in choice B.
In this choice, the same number of atoms of each elements is in both, reactants and products sides, which means that the law of conservation of mass is fulfilled.
The correct answer is choice B.
Which is more reactive: Magnesium or Potassium?
Answers
CH3CHOHCH3 an electrolyte in solution.
Answers
The complete statement is "CH3CHOHCH3 is not an electrolyte in solution." This is further explained below.
What is electrolyte ?Generally,a liquid or gel containing ions that may be broken down by electrolysis, such as the kind used in batteries.
In conclusion, "CH3CHOHCH3 is not an electrolyte in solution," is the whole sentence.
Read more about electrolyte
https://brainly.com/question/14566383
#SPJ1
Balance these equations : ) ……H 2 + …..O 2 —> …. H 2 O…..FeCl 2(s) + ….. H 2 O (1)….> ….FeO (s) + …. HCl(aq)…..C 4 H 8(g) + …..O2(g)……> ….CO 2(g) + ….H 2 O (l)…..NaHCO 3(s) ….> ……Na 2 CO 3 + ….. CO 2(g) +…..H 2 O (g)…..NaOH (aq) + …….NgCl (aq) ….> …..NaCl (aq) + ….Mg(OH) 2(s)
Answers
In order to properly balance an equation, we need to make sure that the same amount of elements on the reactants side matches the number of elements on the products side, we can do that by increasing the number in front of each molecule, the so called stoichiometric coefficient. In the reaction from the question we can properly balance by adding the following stoichiometric coefficients
1. 2 H2 + O2 -> 2 HO2
2. FeCl2 + H2O -> FeO + 2 HCl
3. C4H8 + 6 O2 -> 4 CO2 + 4 H2O
4. ?
5. 2 NaOH + MgCl2 -> 2 NaCl + Mg(OH)2
In your own words, explain what’s happening here. I need help please!!
H2+O2+H2O
Answers
Answer:
Nothing is happening.
Explanation:
As written, nothing is going on. H2+O2+H2O represents a mixture of H2, O2, and H2O. We aren't even given the states, so they may all be gases, liquids/solids, or dissolved gases in a liquid (water).
If we had H2+O2 → H2O, we could say that hydrogen and oxygen are combining to form H2O, water. We should note, however, that the chemical equation is not balanced. There are two oxygen atoms on the reactant side, but only one on the product side. A balanced equation would read:
2H2 + O2 → 2H2O
It would be nice to indicate the physical states, such as:
2H2(g) + O2(g) → 2H2O(l)
Two gases, oxygen and hydrogen, combine to form liquid water.
Also missing from this equation is the energy that may be consumed, or released in this reaction. It would be nice to know, for example, that this reaction releases a lot of energy. Otherwise, we might wind up in the local headlines.
Which variable is unknown until the experiment is performed?
Answers
The variable that is unknown until the experiment is performed is the dependent variable.
In a scientific experiment, variables are classified into two main categories: independent variables and dependent variables. The independent variable is the variable that is intentionally manipulated or changed by the experimenter. It is under the control of the experimenter and is deliberately altered to observe its effect on the dependent variable.
On the other hand, the dependent variable is the variable that is measured or observed as the outcome or response in the experiment. It is the variable that is expected to change in response to the manipulation of the independent variable. The value or behavior of the dependent variable depends on the value or behavior of the independent variable.
Typically, before conducting an experiment, researchers have a hypothesis or an expectation about how the independent variable will affect the dependent variable. However, the actual outcome or result of the experiment, which is observed through the measurement of the dependent variable, remains unknown until the experiment is performed.
The purpose of conducting the experiment is to gather empirical data and observe the changes in the dependent variable to analyze the relationship between the independent and dependent variables.
For more such questions on dependent variable visit:
https://brainly.com/question/28433016
#SPJ8