The order of elements in the modern periodic table is based on an element's
Group of answer choices
atomic number.
atomic mass.
chemical symbol.
name.
Answers
Answer:
atomic number
Explanation:
Related Questions
Lucite contain 59. 9 g C, 8. 06 g H,
and 32. 0 g O. You want to determine the empirical formula. How many mole of C are in the ample?
Answers
The number of moles of Carbon in the sample is 4.99 moles
How to calculate number of moles?The number of moles in a substance can be calculated by dividing the mass of the substance by its molar mass as follows:
moles = mass ÷ molar mass
According to this question, Lucite contain 59.9 g C, 8.06 g H, and 32.0 g O. The moles of C can be calculated as follows:
Molar mass of C = 12g/mol
moles = 59.9g ÷ 12g/mol
moles = 4.99 moles
Learn more about moles at: https://brainly.com/question/26416088
#SPJ1
Why must the final product of a decay series be stable?
a. The daughter isotope of any radioactive decay is stable.
b. No decay chain can emit more than two particles.
c. Each decay results in a net loss of particles.
d. If the daughter isotope is unstable, it will decay.
Answers
Answer:
I think its d
Explanation:
Because isotopes decay due to being unstable so if the daughter isotope is unstable it will just decay till it produces a stable isotope
Answer:
d. If the daughter isotope is unstable, it will decay
Explanation:
got it correct on quiz
The diagram below shows the different phase transitions that occur in matter.
Three bars are shown labeled Solid, Liquid, and Gas. They are connected by arrows labeled 1 to 6. Arrow 1 points from liquid to gas; arrow 2 from solid to liquid, arrow 3 from solid to gas, arrow 4 from gas to liquid, arrow 5 from liquid to solid, and arrow 6 from gas to solid.
Which arrow represents the transition in which dew is formed?
1
2
4
6
Answers
The arrow that represents the transition in which dew is formed would be arrow 4.
How is dew formed?Dew is formed by condensation. Condensation, in itself, involves a transition from a gas phase to a liquid phase.
From the diagram, the arrow that represents a transition from gas to liquid is arrow 4.
Thus, the arrow that represents the transition that leads to the formation of dew would be arrow 4.
More on dew formation can be found here: https://brainly.com/question/23169635
#SPJ1
Answer:
Arrow 4
Explanation:
Why do anticyclones make UHIs stronger?
Answers
Anticyclones are associated with high pressure and subsiding air, which leads to stable atmospheric conditions. This stability can lead to the accumulation of air pollutants, such as particulate matter and nitrogen oxides, within the urban area, resulting in a stronger urban heat island (UHI) effect.
In addition, the subsiding air in anticyclones can cause a reduction in wind speed, which can limit the mixing of air between urban and rural areas, further enhancing the UHI effect.
Finally, clear skies and sunshine associated with anticyclones can lead to more solar radiation being absorbed by urban surfaces, increasing their temperatures and contributing to the UHI effect.
For more question on Anticyclones click on
https://brainly.com/question/27346130
#SPJ11
Number of unpaired electrons in the electronic configuration 1s2 2s2 2p4 is
Answers
Answer:
2 electrons
Explanation:
N2+3H2->2NH3
If 0.600 moles of NH3 were produced. How many moles of H2 is required
Answers
If 0.6 moles of \(NH_3\) were produced from the reaction, the mole of \(H_2\) that is required will be 0.90 moles.
Stoichiometric problemFrom the equation of the reaction, the mole ratio of \(H_2\) that reacts to that of \(NH_3\) that is produced is 3:2.
In other words, for every 1 mole of \(NH_3\) that is produced, 1.5 moles of \(H_2\) is required.
Now, if 0.600 moles of \(NH_3\) were produced from the reaction, the amount of \(H_2\) that is required can be calculated from a simple ratio as thus:
1 mole of \(NH_3\) = 1.5 moles of \(H_2\)
0.600 moles of \(NH_3\) = 1.5 x 0.600/1
= 0.90 moles of \(H_2\)
In other words, if 0.600 moles of \(NH_3\) were produced in the reaction, 0.90 moles of \(H_2\) would be needed to react.
More on stoichiometric problems can be found here: https://brainly.com/question/15047541
#SPJ1
what is the pH of a solution obtained by adding 145 mL of 0.575M HCl to 493ml of HNO3 solution with a pH of 1.39?
Answers
The pH of the resulting solution obtained by adding 145 mL of 0.575 M HCl to 493 mL of HNO₃ solution with a pH of 1.39 is approximately -0.98852.
To find the pH of the resulting solution after mixing HCl and HNO₃, we need to calculate the concentration of the resulting solution and then determine its pH.
Step 1: Calculate the moles of HCl and HNO₃:
Moles of HCl = volume (in liters) × concentration
Moles of HCl = 0.145 L × 0.575 M = 0.083375 moles
Step 2: Calculate the moles of HNO₃:
Moles of HNO₃ = volume (in liters) × concentration
Moles of HNO₃ = 0.493 L × 10^(pH) [concentration is calculated from the pH value]
Moles of HNO₃ = 0.493 L × 10^(1.39) = 6.4661 moles
Step 3: Calculate the total moles of acid:
Total moles of acid = moles of HCl + moles of HNO₃
Total moles of acid = 0.083375 moles + 6.4661 moles = 6.549475 moles
Step 4: Calculate the total volume of the resulting solution:
The total volume of resulting solution = volume of HCl + volume of HNO₃
Total volume of resulting solution = 0.145 L + 0.493 L = 0.638 L
Step 5: Calculate the concentration of the resulting solution:
The concentration of resulting solution = total moles of acid / total volume of solution
Concentration of resulting solution = 6.549475 moles / 0.638 L = 10.262 M
Step 6: Calculate the pH of the resulting solution:
pH = -log10(concentration of H+ ions)
pH = -log10(10.262) = -log10(10) + log10(1.0262) = -1 + 0.01148 = -0.98852
Therefore, the pH of the resulting solution obtained by adding 145 mL of 0.575 M HCl to 493 mL of HNO₃ solution with a pH of 1.39 is approximately -0.98852.
To learn more about pH visit:
brainly.com/question/28227384
In the synthesis of n-butylacetate, you decide to add sodium sulfate to the heating reaction mixture to remove water in order to shift the equilibrium toward ester formation instead of using azeotropic distillation. Why would your efforts not be successful?
Answers
Adding sodium sulfate to the heating reaction mixture in the synthesis of n-butylacetate to remove water and shift the equilibrium towards ester formation would not be successful.
Sodium sulfate is commonly used as a drying agent to remove water from reaction mixtures. However, in the case of the synthesis of n-butylacetate, the addition of sodium sulfate to remove water would not effectively shift the equilibrium towards ester formation.
This is because the formation of water is an inherent part of the esterification reaction, and removing water from the reaction mixture would not have a significant impact on the equilibrium position. Azeotropic distillation is a more effective method to separate the formed ester from water, as it allows for the removal of water without affecting the reaction equilibrium.
To learn more about equilibrium click here: brainly.com/question/30694482
#SPJ11
Determine the [OH−] , Ph, and POH of a solution with a [H+] of 0. 00017 m at 25 °C.
Answers
The answer is \(\underline{12.954}\end{aligned}$$\)
\(\begin{aligned}&\mathrm{pH}=\underline{1.046} \\&{\left[\mathrm{OH}^{-}\right]=\underline{1.11 \times 10^{-13} \mathrm{M}}} \\&\mathrm{pOH}=\underline{12.954}\end{aligned}\)
Given:
\(\left[\mathrm{H}^{+}\right]=0.090 \mathrm{M}=9 \times 10^{-2} \mathrm{M} ; \mathrm{T}\\=25^{\circ} \mathrm{C}$$\mathrm{As}, \mathrm{pH}=-\log \left[\mathrm{H}^{+}\right]$\Rightarrow \mathbf{p H}=-\log \left(9 \times 10^{-2}\right)=\underline{1.046}$$\)
The equation for the self-ionization constant of water is
\($$\mathrm{Kw}=\left[\mathrm{H}^{+}\right]\left[\mathrm{OH}^{-}\right]$$and, $\mathrm{pKw}=\mathrm{pH}+\mathrm{pOH}$\)
Since at room temperature,
\($25^{\circ} \mathrm{C}: \mathrm{Kw}=1.0 \times 10^{-14}, \mathrm{pKw}=14$\)
\(\therefore \mathrm{Kw}=\left[\mathrm{H}^{+}\right]\left[\mathrm{OH}^{-}\right]=1.0 \times 10^{-14}$$\\\Rightarrow\left[\mathrm{OH}^{-}\right]=\left(1.0 \times 10^{-14}\right) \div\left[\mathrm{H}^{+}\right]\\=\left(1.0 \times 10^{-14}\right) \div\left[9 \times 10^{-2}\right]\\=0.111 \times 10^{-12}=1.11$$\times 10^{-13} \mathrm{M}$\)
And
\($$\begin{aligned}&\mathrm{pH}+\mathrm{pOH}=\mathrm{pKw}=14 \\&\Rightarrow \mathrm{pOH}=14-\mathrm{pH}=14-1.046=\underline{12.954}\end{aligned}$$\)
What is Poh and PH linked formula?
Take the negative log of the hydronium ion concentration and use that value to compute pH. Simply subtraction the pH from 14 yields the pOH value. The negative log of the concentration of hydroxide ions should be used to get the pOH. Simply subtraction 14 from pOH yields the pH.So the more about Poh and PH linked formula.
https://brainly.com/question/16867635
#SPJ4
Determine the number of atoms of O in 32.3 moles of Al₂(SO₄)₃.
Answers
387.6 number of atoms of oxygen are in 32.3 moles of Al₂(SO₄)₃.
What do you mean by the mole concept ?
Mole is the amount of substance in a chemical system that contains as many elementary entities as there are atoms in exactly 12 grams of the carbon−12 isotope.
The mole concept is a convenient method of expressing the amount of a substance.
To calculate the the number of atoms of O in 32.3 moles of Al₂(SO₄)₃-:
The chemical formula for aluminum carbonate, Al₂(SO₄)₃ , indicates that in one mole of the compound there are twelve moles of oxygen atoms.
Now,
=22.3 m o l Al₂(SO₄)₃× 12 m o l O atoms/1 m o l Al₂(SO₄)₃
= 387.6 m o l of O atoms
Hence, 387.6 number of atoms of oxygen are in 32.3 moles of Al₂(SO₄)₃.
Learn more about mole concept ,here:
https://brainly.com/question/22540912
#SPJ1
which two events are apart of the rock cycle
Answers
Answer:
The key processes of the rock cycle are crystallization, erosion and sedimentation, and metamorphism.
Explanation:
mark me brainliest!!

Answer:
Earths heat and hydrological cycle
Explanation:
A student researches how two types of rocks are formed and records the information below.
Rock Formation
Rock 1: Formed by cooling magma
Rock 2: Formed by compacting and cementing particles together
The student claims that Rock 2 is more likely to contain a fossil than is Rock 1
Which statement BEST describes the students claim?
There wasn’t a science option :(
Answers
Answer: Based on Rock Formation, the student's judgment that Rock 2 is more likely to contain a fossil than Rock 1 is appropriate.
Explanation:
Rocks are classified into three types:
Igneous rocks, metamorphic rocks, and sedimentary rocks.
Rock 1 is an Igneous Rock.
When a fiery, molten rock crystallizes and hardens, it forms igneous rocks (from the Latin root word fire).
Rock 2 is a Sedimentary Rock
At standard surface temperatures, sedimentary rock is developed at or near the Earth's surface by the build-up and lithification of sediment (detrital rock) or by precipitation from suspension (chemical rock).
Under low temperatures and pressure, rock deposits produce sedimentary rocks in the ocean and on the Earth's crust. Plants and animals die and are deposited in a sedimentary deposit.
The following are the reasons why sedimentary rocks are the only rock types that hold fossils:
The burial of deceased plants and animals by additional sediment results in the build-up of minerals from water in bones, which leads to the development of fossils.
As a result, sedimentary rocks are the only rock types that retain fossils.
To learn more about Rocks
https://brainly.com/question/19709356
What happens to Hydrogen molecules after the light reaction in photosynthesis? Hydrogen is released into the atmosphere Hydrogen bonds with Carbon Dioxide to form glucose Hydrogen bonds with Oxygen to form water.
Answers
Photosyntheis is a biochemical reaction that combines chemicals with biological species to produce energy. Atoms of hydrogen bond with carbon to form glucose.
What are the reactants and the products of the light reaction?Carbon dioxide and hydrogen are the reactants that are used from the environment by the plants to make energy. Glucose and oxygen are the products.
The hydrogen atoms from the environment are absorbed by the plants and are combined with the carbon dioxide to yield glucose or sugar molecules for energy.
Therefore, option B. hydrogen and carbon dioxide react to produce glucose.
Learn more about photosynthesis here:
https://brainly.com/question/1388366
(42.7g +0.259g) / (28.444mL x 12.367)
Answers
(SOLUTION)
Mass/volume = total mass/ total volume
Now:
Total mass= 42.95 g
Total volume =351.8mL
Put these values in above equation;
Mass/volume = 42.95/351
“”. = 0.122 g/mL
balance the following skeleton equations Mg+02---->MgO
Answers
Answer: \(2Mg+O_2\rightarrow 2MgO\)
Explanation:
Skeletal equations are defined as the equations in which formula are used to indicate the chemicals that are involved in a chemical reaction.
According to the law of conservation of mass, mass can neither be created nor be destroyed. Thus the mass of products has to be equal to the mass of reactants. The number of atoms of each element has to be same on reactant and product side. Thus chemical equations are balanced.
Thus the balanced chemical equation will be :
\(2Mg+O_2\rightarrow 2MgO\)
A characteristic property of matter that can be used to identify a substance is?

Answers
Answer:
Volume
Explanation: Volume is a characteristic property of matter that can be used to identify a substance.
If you begin with the highest amount of available entropy, which phase change would represent a decrease in entropy?
Answers
Phase changes involving an increase in the degree of molecular disorder typically correspond to an increase in entropy.
Conversely, phase changes that decrease the degree of molecular disorder are associated with a decrease in entropy.
At a given temperature and pressure, the sequence of phase changes that represents an increase in the degree of molecular disorder is:
solid → liquid → gas
Therefore, the sequence of phase changes that represents a decrease in the degree of molecular disorder, and hence a decrease in entropy, is:
gas → liquid → solid
For example, when water vapor condenses to form liquid water, the degree of molecular disorder decreases, and hence entropy decreases.
in some cases, the phase change from a gas to a solid may bypass the liquid phase altogether, as in the case of deposition, where a gas transforms directly into a solid.
Learn more about entropy and phase changes here:
https://brainly.com/question/16718133
#SPJ4
What does interdisciplinary science mean?
Answers
Answer:
Something that's interdisciplinary covers more than one field of study. If you take an interdisciplinary science and literature class, you might read a science fiction novel and then explore the scientific ideas behind it. ... Interdisciplinary means between fields, but they don't have to be unrelated disciplines.
Explanation:
hope this helps:)
Farmers recently have started to use a lot of fertilizer on farms. Extra fertilizer runs off the farms, into the rivers, and out into the oceans. Which of the following would be a likely outcome of this?
A) Animals will use the fertilizer to grow.
B) Kelp on the bottom of the ocean will grow.
C) Coral reefs will grow faster.
D) Algae will grow, causing algae blooms.
Answers
answer:
d) algae will grow, causing algae blooms
explanation:
changes in the environment create algae blooms. (ie. excessive use of fertilizer)
https://quizi-zz.com/admin/quiz/5d78f527bbbbcc001ac835d2/ecosystems
good luck :)
i hope this helps
have a great day!
Extra fertilizer runs off the farms, into the rivers, and out into the oceans. Algae will grow, causing algae blooms. Therefore, the correct option is option D.
What is fertilizer?Any substance of either synthetic or natural origin applied to soil or plant tissues to provide plant nutrients is referred to as a fertilizer or fertiliser. It's possible to distinguish fertilizers from liming agents and other non-nutrient soil additives. There are numerous natural and man-made sources of fertilizer.
With the exception of the sporadic inclusion of supplements such rock flour for micronutrients, fertilization for the majority of modern agricultural techniques concentrates on the three basic macronutrients of nitrogen (N), phosphorous (P), plus potassium (K). Farmers recently have started to use a lot of fertilizer on farms. Extra fertilizer runs off the farms, into the rivers, and out into the oceans. Algae will grow, causing algae blooms.
Therefore, the correct option is option D.
To know more about fertilizer, here:
https://brainly.com/question/11085567
#SPJ3
gas exchange occurs in what structure of the respiratory zone
Answers
Gas exchange occurs in the structure called the alveoli in the respiratory zone. The respiratory zone is the region of the lungs where actual gas exchange takes place between the respiratory surfaces and the blood.
The alveoli are small, thin-walled sacs located at the end of the respiratory bronchioles. They are surrounded by a network of capillaries, where oxygen from the inhaled air diffuses into the bloodstream, and carbon dioxide, a waste product of cellular respiration, diffuses out of the bloodstream into the alveoli to be exhaled.
The alveoli provide a large surface area and thin walls lined with a moist layer of surfactant to facilitate efficient gas exchange. This structure allows for the diffusion of oxygen from the alveoli into the blood and the diffusion of carbon dioxide from the blood into the alveoli. This exchange of gases is essential for the oxygenation of the blood and the removal of carbon dioxide, supporting the body's respiratory function.
To learn more about alveoli
https://brainly.com/question/29435999
#SPJ11
PLEASE HELP ITS DUE SOON PLEASE ILL GIVE BRAINLIESTTT

Answers
Answer:
b. tendons connected muscles to bone.
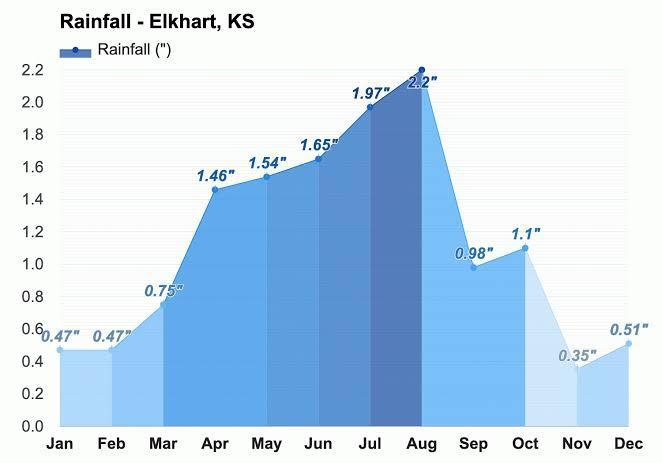
create a bar graph that shows the total yearly precipitation for elkhart kansas
Answers
The total yearly precipitation for elkhart kansas is attached in the form of graph below.
What is precipitation?Precipitation is defined as any liquid or frozen water which forms in the atmosphere and then gets received on Earth.It is one of the most important steps of the water cycle.
Precipitation takes place in form of clouds when water vapor gets accumulated in clouds and they get bigger and heavy, when the clouds become heavy enough they fall to the land in the form of rain.f a cloud is present at higher altitudes , the water present in the clouds freezes and fall to the ground in form of snow,hail.
Learn more about precipitation ,here:
https://brainly.com/question/18109776
#SPJ1
A sample of gas contains 6.25 × 10-3 mol in a 500.0 mL flask at 265°C.
Solve the problem for the pressure of oxygen.
in kPa plz
Answers
Answer:
The pressure of gas in kilopascals is 55.9
Explanation:
variables given :
volume
temperature
moles
unknown variables :
pressure
Select the correct algebraic form for the equation to be used for this calculation.
p= nRT/V
Solve the problem for the pressure of oxygen.
Answer:
55.9 kPaa 20.0 ml sample of glycerol has a mass of 25.8 grams. what is the density of glycerol in ounces/quart?
Answers
Glycerol has a density of ounces/quart equal to. The mass of a sample of glycerol is grams. 1 ounce equals 28.4 grams.
What is Glycerol?Glycerol is a colourless, odourless, thick liquid generated from both animal and plant fats. It is also known as glycerin or glycerine. It is always found in a wide range of goods, including medications, personal hygiene products, food, and drinks. It is a natural moisturizer, fluid, and lubricant and is a key component of many fats.
Glycerol – a fat or a sugar?Glycerin is a type of carbohydrate known as a polyol, along with other sugar alcohols including sorbitol and erythritol. Glycerin feels sweet, just as sugar alcohols, which I've already discussed. Many living creatures require the sugar alcohol glycerol to survive. It is a part of lipids like phospholipids and glycerides, among others. Glycerol joins with fatty acids to create glycerides, which can act as an energy source.
To know more about glycerol, visit
brainly.com/question/29240183
#SPJ1
Find the density of a liquid if the mass is 30 grams and the volume is 10 ml. If the density of a liquid is 2 g/ml and the volume is 30 ml, what is the mass of the
liquid? If the density of the liquid is 5 g/ml and the mass is 50 grams, what is the volume of the liquid?
g/ml.
The density of the liquid is
The mass of the liquid is
The volume of the liquid is
grams.
ml.
Answers
Answer:
The density of liquid is 3 g/mL.
The mass of liquid is 60 g.
The volume of liquid is 10 mL.
Explanation:
Given data:
Density of liquid = ?
Mass of liquid = 30 g
Volume of liquid = 10 mL
Solution:
d = m/v
d = density
m = mass
v = volume
Now we will put the values in formula.
d = 30 g/ 10 mL
d = 3 g/mL
The density of liquid is 3 g/mL.
2nd:
Given data:
Density of liquid = 2 g/mL
Volume of liquid = 30 mL
Mass of liquid = ?
Solution:
d = m/v
2 g/mL = m/ 30 mL
m = 2 g/mL×30 mL
m = 60 g
The mass of liquid is 60 g.
3rd:
Given data:
Density of liquid = 5 g/mL
Mass of liquid = 50 g
Volume of liquid = ?
Solution:
d = m/v
5 g/mL = 50 g/ v
v = 50 g/5 g/mL
v = 10 mL
The volume of liquid is 10 mL.
WILL MARK BRANLIEST HELP PLEASE
When copper sulfate and zinc metal interact, one atom of zinc replaces one atom of copper while the sulfate, made of 5 atoms, is unchanged. How many atoms are there before and after the interaction?
before: 5 atoms; after: 5 atoms
before: 6 atoms; after: 7 atoms
before: 6 atoms; after: 6 atoms
before: 7 atoms; after: 7 atoms
Answers
when water is heated to transform from a liquid to a gas
Answers
Answer:
evaporation
Explanation:
When water is heated, it evaporates. The molecules move and vibrate so quickly that they escape into the atmosphere as molecules of water vapor. Heat from the sun, or solar energy, powers the evaporation process.
like any other equilibrium constant, kw is also affected by temperature. the kw at 75 degrees celsius is 1.995 x 10⁻¹³. what is the poh of water at this temperature?
Answers
Kw of water is the product concentration of H+ and concentration OH- ions . The OH - concentration for a Kw of 1.995 ×10⁻¹³ is 4.46 × 10⁻⁷. Hence, the pOH is 6.35.
What is pOH ?pOH of a solution is the measure of its OH- ion concentration as pH for the H+ ion concentration. pOH is the negative logarithm of the molar concentration of OH- ions.
Like pH, pOH also indicates the acidity or basicity of the solution.
The equilibrium constant Kw of water = [OH-] [H+]
for neutral water = [H+] = [OH-]
Then [OH-]² = kw = 1.995 ×10⁻¹³
[OH-] = √(1.995 ×10⁻¹³) = 4.46 × 10⁻⁷
pOH = - log [OH-]
= - log (4.46 × 10⁻⁷)
= 6.35.
Therefore, pOH of water at 75 degree celsius is 6.35.
Find more on pOH:
https://brainly.com/question/17144456
#SPJ1
When is the ground considered saturated?
when there are air pockets between rock particles
O when all spaces between rock particles are filled with water
when most of the space between rock particles is filled with water
O when there is no space between any rock particles
Answers
Answer:
when all the spaces between rock particles is filled with water
Two atoms bonded together will remain some distance apart, minimizing the Question 1 options: A) potential energy of the bond. B) bond distance. C) number of valence electrons in the bond. D) partial charge of the bond. Question 2 (5 points) BeH2 has no lone pairs of electrons. What's the structure of this molecule? Question 2 options: A) Tetrahedral B) Bent C) Octahedral D) Linear Question 3 (5 points) In KCl, how are the valence electrons distributed? Question 3 options: A) The electrons are transferred from K to Cl. B) The electrons are unequally shared between K and Cl, forming a polar covalent bond. C) The electrons are shared between many K and Cl ions, creating a "sea of electrons." D) The electrons are equally shared between K and Cl, forming a covalent bond. Question 4 (5 points) Chlorine can bond with fluorine to form ClF. Chlorine can also bond with lithium to form LiCl. Which compound will have a greater partial charge? Question 4 options: A) Both compounds will have the same partial charge. B) ClF C) LiCl D) Neither compound will have partial charge. Question 5 (5 points) Which of the following elements will not form a polar covalent bond with oxygen? Question 5 options: A) Hydrogen B) Oxygen C) Sodium D) Fluorine Which process is used to produce gases from solutions of salts dissolved in water or another liquid? Question 6 options: A) Electrolysis B) Polar covalent bonding C) Ionic bonding D) Metallic bonding Question 7 (5 points) Saved A chemical reaction has the equation AgNO3 (s) + NaCl (s) → AgCl (s) + NaNO3 (s). What type of reaction occurs between AgNO3 and NaCl? Question 7 options: A) Decomposition B) Double displacement C) Single displacement D) Synthesis
Answers
Answer:
1) potential energy of the bond.
2) Linear
3) The electrons are transferred from K to Cl.
4) ClF
5) Oxygen
6) Electrolysis
7) Double displacement
Explanation:
As two atoms approach each other in a bonding situation, the potential energy of the bond is minimized as the internuclear distance of the bonding atoms decreases.
BeH2 has two electron domains and the central beryllium atom is sp2 hybridized. According to valence shell electron pair repulsion theory. A molecule having two regions of electron density will lead to a linear molecule.
KCl is an ionic compound hence there is a transfer of electrons from K(metal) to Cl(nonmetal).
ClF has partial charges because it contains a polar covalent bond. The partial charges arise from the dipole within the molecule. LiF is a pure ionic compound formed by transfer of electrons from Li to F. The species possess full and not partial charges.
When an oxygen atom bonds with another oxygen atom, what has been formed is a homonuclear covalent bond. Since the electro negativity of the both atoms is exactly the same, a pure covalent bond is formed. Recall that polar covalent bonds are formed when there is a significant electro negativity difference between the bonding atoms.
When direct current is passed through certain salt solutions during electrolysis, gases may be evolved and collected at the appropriate electrodes.
A double-replacement reaction is a reaction in which the cations and anions present in two different ionic compounds that are reacting together exchange their positions to form two new compounds on the product side. For instance, look at the reaction shown in question 7 as a typical example of this;
AgNO3 (s) + NaCl (s) → AgCl (s) + NaNO3 (s).