The muscle responsible for the majority of a particular movement is known as the
a. prime mover
b. synergist
c. antagonist
d. fixator
Answers
Answer:
A.prime mover is Answer.
Explanation:
Prime mover. the muscle responsible for most of the movement in a group of muscles ; called the chief muscle.
I hope it's helpful!
Related Questions
2. a student is given 5.00 ml of an hcl solution of unknown concentration. she divides the sample into five 1.00 ml samples. she then titrates each separately with 0.100 m naoh. the average volume of naoh solution used to reach the endpoint is 15.6 ml. what was the concentration of hcl in the 5.00 ml sample ?
Answers
The concentration of HCl in the 5.00 ml sample is 3.12 M.
The student titrated five 1.00 ml samples of the HCl solution with 0.100 M NaOH. The average volume of NaOH solution used to reach the endpoint was 15.6 ml. This means that 15.6 ml of 0.100 M NaOH was required to neutralize 5.00 ml of HCl solution. The molarity of HCl can be calculated as follows:
Molarity of HCl = (Volume of NaOH / Volume of HCl) * Molarity of NaOH
Molarity of HCl = (15.6 ml / 5.00 ml) * 0.100 M
Molarity of HCl = 3.12 M
To learn more about titration click brainly.com/question/31271061
#SPJ11
What is the mass of
6.45 x 1025 atoms Cu? The molar mass
of Cu is 63.55 g/mol.
A. 3.88 x 104⁹ g Cu
C. 107 g Cu
B. 0.593 g Cu
D. 6,810 g Cu

Answers
The mass of 6.45 x 10²⁵ atoms of Cu is 6810g (option D).
How to calculate mass?The mass of a substance can be calculated by multiplying the no of moles in the substance by its molar mass as follows:
mass = no of moles × molar mass
However, the number of moles in the substance needs to be calculated first as follows:
no of moles = 6.45 x 10²⁵ ÷ 6.02 × 10²³
no of moles = 1.07 × 10² moles
mass of Cu = 107 moles × 63.55g/mol
mass of Cu = 6808.9
mass of Cu is approximately 6810g
Learn more about mass at: https://brainly.com/question/7219907
#SPJ1
2H₂ + O₂ → 2H₂O
Convert grams of H₂ to grams of H₂O.
a. 44,680 g H₂O
b. 5,000 g H₂O
c. 345676543 g H₂O
d. 3335 g H₂O
Answers
Answer:
500100 benswuer kers olá Marilene
The mass of H₂O is 44,680 g for the given reaction 2H₂ + O₂ → 2H₂O. Therefore, the correct option is option A.
What is mass?In physics, mass is a quantitative measurement of inertia, a basic characteristic of all matter. It essentially refers to a body of matter's resistance to changing its speed or location in response to the force that is applied. The change caused by either an applied force is smaller the more mass a body has.
The kilogramme, which is defined approximately equal to 6.6 × 10⁻³⁴ joule second in terms of Planck's constant, is the unit of mass inside the Worldwide System of Units (SI). A joule is equivalent to one kilogramme multiplied by one square metre per second. The mass of H₂O is 44,680 g for the given reaction 2H₂ + O₂ → 2H₂O.
Therefore, the correct option is option A.
To know more about mass, here:
https://brainly.com/question/28704035
#SPJ7
a transition metal ion, x3 , has the electronic configuration [ar] 3d4. determine the atomic number of element x.
Answers
The element is Manganese (Mn).
The transition metal electronic configuration in the +3 oxidation state is [Ar]3d4 The neutral element electronic configuration is [Ar]4s 2 3d 5, Atomic number should be =25. Therefore the element is Manganese(Mn).
The atomic number is the number of a chemical element in the periodic table. Elements are arranged according to the increasing number of protons in the nucleus. Therefore, the number of protons, which is always equal to the number of electrons in neutral atoms, is also the atomic number. The atomic number is simply the number of protons in an atom. For this reason, it is sometimes called the proton number.
Learn more about The atomic number here:- https://brainly.com/question/621740
#SPJ4
A student must make a buffer solution with a pH of 3.50. Determine which weak acid is the best option to make a buffer at the specified pH. acetic acid, Ka = 1.75 x 10-5 5.00 M propionic acid, Ka = 1.34 x 10-5 3.00 M formic acid, Ka = 1.77 x 10-4 2.00 M phosphoric acid, Ka = 7.52 x 10-;3 1.00 M Incorrect
Answers
The pKa of acetic acid is 4.75. Since pH is less than pKa, this implies that acetic acid is present in greater amounts than its conjugate base, and therefore acetic acid is the best weak acid to make a buffer solution with a pH of 3.50. Hence, the correct option is acetic acid,
Ka = 1.75 × 10−5, 5.00 M,
which makes it the best option to make a buffer at the specified pH.
A student must make a buffer solution with a pH of 3.50. To determine which weak acid is the best option to make a buffer at the specified pH, first we need to calculate the pH of the buffer solution.What is a buffer solution?A buffer solution is a solution that resists changes in pH even when a small amount of acid or base is added. Buffers are prepared by mixing weak acids or bases with their corresponding conjugate base or acid, respectively. A buffer's pH is given by the pKa, and it is determined by the ratio of the weak acid and its conjugate base in the buffer solution.The pH of the buffer solution can be calculated by using the formula:
pH = pKa + log([A-]/[HA])
Where pH is the required pH of the buffer solution. A- and HA are the concentrations of the conjugate base and weak acid, respectively. pKa is the negative logarithm of the acid dissociation constant (Ka).The student is asked to prepare a buffer solution with a pH of 3.50. The acid should be weak so that it does not completely dissociate. The given options are acetic acid, propionic acid, formic acid, and phosphoric acid. The best weak acid to use to prepare a buffer solution with a pH of 3.50 would be acetic acid.Acetic acid, CH3COOH is a weak acid, and its
Ka is 1.75 × 10−5.
Its conjugate base is CH3COO−.To make a buffer solution, we mix a weak acid and its conjugate base in a specific ratio, which is usually close to 1:1. So, we should look for an acid that has a pKa closest to the target pH of 3.50. The pKa of acetic acid is 4.75. Since pH is less than pKa, this implies that acetic acid is present in greater amounts than its conjugate base, and therefore acetic acid is the best weak acid to make a buffer solution with a pH of 3.50. Hence, the correct option is acetic acid,
Ka = 1.75 × 10−5, 5.00 M,
which makes it the best option to make a buffer at the specified pH.
To know more about acetic acid visit:
https://brainly.com/question/4300470
#SPJ11
What the anode , cathode and the electrolyte of a cell tha t you might use to electrolyte a spoon made from iron with silver?
Answers
The silver coating on the spoon is produced. When electrolyzing a spoon made from iron with silver, the anode, cathode, and electrolyte that can be used are as follows:
Anode: The anode is a negatively charged electrode, usually made of metal or graphite, that releases electrons during electrolysis. It is made of pure silver.Cathode: The cathode is a positively charged electrode that receives electrons during electrolysis. It is made of iron.Electrolyte: The electrolyte is a solution that conducts electricity and contains ions that can be reduced or oxidized. The electrolyte used for this process is a solution of silver nitrate (AgNO3) in water.The silver ion (Ag+) moves from the anode to the cathode through the electrolyte. At the cathode, it accepts an electron, reducing it to metallic silver (Ag). Fe(s) is oxidized to Fe2+(aq) ion at the anode, while Ag+ ions are reduced to Ag(s) at the cathode. Therefore, the silver coating on the spoon is produced.For such more questions on silver coating
https://brainly.com/question/29736740
#SPJ8
Which scenario would be classified as potential energy?
A car driving up a hill
A car sitting on top of a hill
The plane flying the skydiver to the jump mark
Answers
Answer:
a car sitting on top of a hill
Explanation:
if it's sitting it has no kinetic energy
A sample of gas (1.9 mol) is in a flask at 21 °C and 697 mmHg. The flask is opened and more gas is added to the flask. The new pressure is 795 mmHg and the temperature is now 26 °C. There are now __________ mol of gas in the flask.
Answers
Answer:
The new moles of the gas in the flask is 2.13 moles.
Explanation:
Given;
number of moles of gas, n = 1.9 mol
temperature of the gas, T = 21 °C = 21 + 273 = 294 K
pressure of gas, P = 697 mmHg
volume of gas, V = ?
Apply ideal gas law;
PV = nRT
Where;
R is gas constant, = 62.363 mmHg.L / mol. K
V = nRT / P
V = (1.9 x 62.363 x 294) / 697
V = 49.98 L
New pressure of the gas, P = 795 mmHg
New temperature of the gas, T = 26 °C = 273 + 26 = 299 K
New moles of the gas, n = ?
Volume of the gas is constant because volume of the flask is the same when more gas was added.
n = PV / RT
n = (795 x 49.98) / (62.363 x 299)
n = 2.13 moles
Therefore, the new moles of the gas in the flask is 2.13 moles.
which of the following disinfectants acts by disrupting the plasma membrane? group of answer choices aldehydes soaps heavy metals halogens bisphenols
Answers
Soaps act by disrupting the plasma membrane of microorganisms. They are able to do so because they have amphipathic properties, which means that they have both hydrophilic (water-loving) and hydrophobic (water-fearing) properties.
The hydrophobic end of the soap molecule can insert itself into the plasma membrane of a microorganism, while the hydrophilic end remains in contact with the surrounding water. This causes the membrane to become disrupted, leading to the death of the microorganism. Therefore, the correct answer is soaps.
The disinfectant that acts by disrupting the plasma membrane is bisphenols.
For more question on bisphenols
https://brainly.com/question/14446118
#SPJ11
During which phase of the moon does a lunar eclipse occur?
A
first quarter
B
full
C
last quarter
D
new
Answers
A lunar eclipse can occur only at full moon. A total lunar eclipse can happen only when the sun, Earth and moon are perfectly lined up — anything less than perfection creates a partial lunar eclipse or no eclipse at all.
stars with masses less than ____ produce most of their energy via the proton-proton chain.
Answers
The nuclear fusion process that powers stars is dependent on the temperature and pressure of the star's core. For stars with lower masses, the temperature and pressure in the core are not high enough to sustain nuclear fusion via the CNO cycle, which requires higher temperatures and densities.
The proton-proton chain is a fusion process that occurs in stars that are not massive enough to support the CNO cycle. In this process, four protons are fused together to form a helium-4 nucleus (also known as an alpha particle), releasing energy in the form of gamma rays and neutrinos.
The proton-proton chain can occur in two main forms: the proton-proton I (pp I) chain and the proton-proton II (pp II) chain. The pp I chain is the dominant process in stars with masses less than about 1.2 times the mass of the Sun. In this process, two protons combine to form a deuterium nucleus, which then combines with another proton to form a helium-3 nucleus. Two helium-3 nuclei then combine to form a helium-4 nucleus, releasing energy in the process.
In stars with masses greater than about 1.2 times the mass of the Sun, the CNO cycle becomes the dominant process for energy generation. In this process, carbon, nitrogen, and oxygen (CNO) act as catalysts to convert hydrogen into helium. The CNO cycle requires higher temperatures and densities than the proton-proton chain, and is more efficient at producing energy in larger stars.
For more questions like masses visit the link below:
https://brainly.com/question/28268576
#SPJ11
Imagine you are bitten by a poisonous snake. You recognize the snake as one that produces venom that cleaves the fatty acids from the glycerol moiety of glycerophospholipids. Which of the following phospholipase inhibitors will most likely inactivate the snake venom? Choose one:
A. phospholipase Cinhibitor
B. an anticoagulant
C. phospholipase A2 inhibitor
D. phospholipase D inhibitor
Answers
Answer:
Phospholipase A2 is the enzyme that cleaves the fatty acids from the glycerol moiety of glycerophospholipids. So, a phospholipase A2 inhibitor would most likely inactivate the snake venom. The correct answer is C. phospholipase A2 inhibitor.
Count the atoms in this common formula for the explosive TNT
2C7H5(NO2)3
Answers
Answer:
Explanation:
7 carbon atoms, 6 hydrogen atoms,9 NO2 atoms
7+6+9=22
2(22)=44
44 atoms
In most mirrors, the virtual image appears to come from behind the mirror. True False
Answers
Answer
False
Explanation:
what is the chem formula for chromium (V) chloride?
Answers
...............
PLEASE help me 10 points

Answers
Answer: A.) unsaturated
Explanation:
if a sample at equilibrium contained 0.051 m h2, 0.087 m s2, and 0.97 m h2s, what would keq be for this reaction at that temperature? (2 points)
Answers
The equilibrium constant (Kₑq) for the reaction at the given temperature would be 0.051 × 0.087 / 0.97².
The equilibrium constant (Kₑq) is a measure of the extent of a chemical reaction at equilibrium. It is calculated by taking the ratio of the concentrations of the products to the concentrations of the reactants, each raised to the power of their respective stoichiometric coefficients.
In this case, the reaction involves the gases H₂, S₂, and H₂S. The given concentrations are 0.051 M for H₂, 0.087 M for S₂, and 0.97 M for H₂S. The stoichiometric coefficients of the reactants and products are not provided, so assuming a balanced equation, we can write the equilibrium constant expression as Kₑq = [H₂][a] [S₂[b] / [H₂S][c], where a, b, and c are the stoichiometric coefficients.
By plugging in the given concentrations, the expression for Kₑq becomes Kₑq = 0.051 × 0.087 / 0.97². Evaluating this expression gives the equilibrium constant for the reaction at the specified temperature.
learn more about equilibrium constant here
https://brainly.com/question/31321186
#SPJ11
3. Predict Suppose the chef used two silver
pans instead, but one was three times the
mass of the other. How would the energy
change of the two pans compare?
Answers
The specific heat capacity of a substance is the amount of heat needed to elevate it by 1 degree kelvin per gram. The easier it is for something to heat up, then, the smaller the heat capacity. We would suppose that a cook in a hurry would want a pan that heats up more quickly and would choose one with a lesser heat capacity.
What would be the difference in the two pans' energy changes?0.385 for copper and 0.900 for aluminum.
Despite using non-SI units, we can still compare using it. It is obvious to him that copper has a lower specific heat, so he will pick that.
In case you're curious, you can also approach this issue from the perspective of heat conduction. To do this, look up the thermal conductivities of each material, and then apply Fourier's rule of heat conduction to determine that Copper would be best.
To know more about specific heat visit:-
https://brainly.com/question/11297584
#SPJ1
why oxidation and reduction occur together ?
Answers
Answer:
a substance cannot gain electron without taking them out from another substance.
Hope this helps
What does this weather map symbol represent, and what is its meaning?

Answers
Answer:
D is the correct answer to your question
how high must a column of water be to exert a pressure equal to that of a 760.0 mm column of mercury? (the density of water is 1.00 g/ml, whereas that of mercury is 13.6 g/ml).
Answers
10336mm high must a column of water be to exert a pressure equal to that of a 760.0 mm column of mercury
76× 13.6×g =h×1×g
h= 76×13.6
h= 1033.6 cm
h= 10336 mm
Mercury is a metal and the eighty-first element on the periodic table. This substance is in the +2 oxidation state.The 16th element on the periodic table, sulphur, is classified as a non-metal. This substance is in the second state of oxidation.Ionic compound is what results from the reaction of a metal and a non-metal. A compound that is created through the complete transfer of electrons from one atom to another is referred to be an ionic compound.
how high must a column of water be to exert a pressure equal to that of a 760.0 mm column of mercury? (the density of water is 1.00 g/ml, whereas that of mercury is 13.6 g/ml).
Learn more about mercury here:
https://brainly.com/question/3116985
#SPJ4
What is the name of the molecule below?
O A. 1-ethyne
B. 1-butyne
C. 2-butyne
D. 2-ethyne
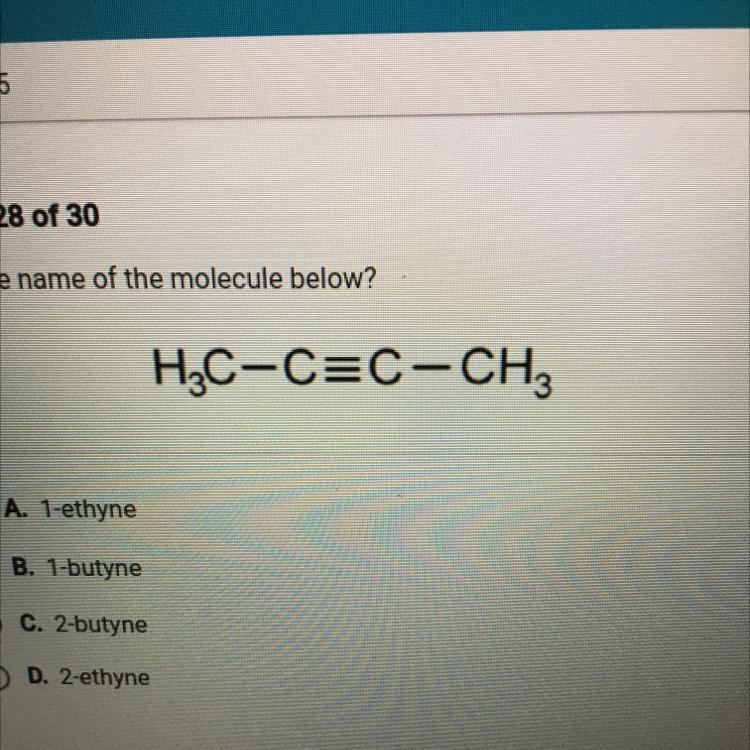
Answers
Answer:
C.) 2-butyne
Explanation:
Since the molecule has 4 central carbons, it has the prefix but-.
Since the molecule has a triple bond between central carbons, it has an ending of -yne.
Since the triple bond starts on the second carbon, it has a 2 - prefix.
For the following reaction Kc = 1.0 × 10^-5 at 30 °C.2NOCl(g) <====> 2NO(g) + Cl2(g)Which relationship is correct at equilibrium at this temperature?A. The concentration of NO equals the concentration of NOCl.B. The concentration of NOCl is double the concentration of Cl2.C. The concentration of NOCl is much greater than the concentration of Cl2.D. The concentration of NO is much greater than the concentration of NOCl.
Answers
c) The concentration of NOCl is much greater than the concentration of Cl2 relationship is correct at equilibrium at this temperature.
Chemical equilibrium refers to the situation in a chemical reaction where both the reactants and products are present in concentrations that have no further propensity to change over time, preventing any discernible change in the system's properties. When the forward reaction and the reverse reaction move forward at the same speed, this condition occurs. The forward and backward responses typically have equal, if not zero, reaction rates. The concentrations of the reactants and products do not alter on a net basis as a result. Dynamic balance is the name given to such a situation.
To know more about equilibrium:
https://brainly.com/question/3920294
#SPJ4
At point R, rocks melt underground to form magma. Which is another process that contributes to the formation of rock at point R?
compacting of sediments on the mountain
cooling as the lava runs down the mountain
weathering of the mountain from the environment
eroding of different rocks at the base of the mountain
Answers
Another process that contributes to the formation of rock at point R is the:
B. Cooling as the lava runs down the mountainAccording to the given question, we need to find another process which can help with the rock formation at point R where the rock melts underground to form magma.
As a result of this, another process through which makes a significant contribution to the formation of rock at point R is the cooling of the lava as the magma which has been melted runs down the mountain.
Therefore, the correct answer is option B
Read more here:
https://brainly.com/question/14886424
2. Which element below would be the best choice when creating a covalent molecule with an
Oxygen atom?
A. Na
B. Mg
C. Ne
D. O
Answers
What part of a microwave oven protects humans against radiation?
plastic outer covering
magnetrons
metal shields
glass doors
Answers
Answer:
The metal shields
Explanation:
got it right on the quiz
the time taken for half the radioactive nuclei in a sample to decay is called the of the nuclide. this value is characteristic of a specific and is not dependent on the number of nuclei present. true or false?
Answers
The time taken for half of the radioactive nuclei in a sample to decay is called the half-life of the nuclide. This value is indeed characteristic of a specific nuclide and is not dependent on the number of nuclei present.
The statement is true. The half-life of a radioactive nuclide refers to the time it takes for half of the radioactive nuclei in a sample to decay. It is a fundamental property of a specific nuclide, meaning that each nuclide has its own unique half-life value. The half-life is constant for a given nuclide and is not influenced by the number of nuclei present in the sample.
The concept of half-life is crucial in understanding radioactive decay and its applications in various fields like radiometric dating, nuclear physics, and medical imaging. The half-life allows scientists to predict how long it will take for a given amount of radioactive material to decay by half. Regardless of the initial amount of radioactive nuclei, the proportion that decays remains the same for each half-life interval.
This property makes the half-life a reliable measure for determining the rate of decay and estimating the age or activity of a radioactive substance.
Learn more about radioactive nuclide here:
https://brainly.com/question/2872076
#SPJ11
Given the standard enthalpy changes for the following two reactions
Given the standard enthalpy changes for the following two reactions:
(1) 2C(s) + 2H2(g)C2H4(g)...... ΔH° = 52.3 kJ
(2) 2C(s) + 3H2(g)C2H6(g)......ΔH° = -84.7 kJ
what is the standard enthalpy change for the reaction:
(3) C2H4(g) + H2(g)C2H6(g)......ΔH° = ?
Answers
The standard enthalpy change for reaction (3) is 117.1 kJ.
The standard enthalpy change for reaction (3) can be calculated by using the enthalpy changes of reactions (1) and (2) and applying Hess's Law.
To do this, we need to manipulate the given equations so that the desired reaction (3) can be obtained.
First, we reverse reaction (1) to get the formation of C2H4(g) from C2H6(g):
C2H4(g)C2H6(g) ΔH° = -52.3 kJ
Next, we multiply reaction (2) by 2 and reverse it to obtain 2 moles of C2H6(g) reacting to form 3 moles of H2(g):
2C2H6(g)2C(s) + 3H2(g) ΔH° = 169.4 kJ
Now, we add the two modified equations together:
C2H4(g)C2H6(g) ΔH° = -52.3 kJ
2C2H6(g)2C(s) + 3H2(g) ΔH° = 169.4 kJ
When adding these equations, the C2H6(g) on the left side cancels out with the C2H6(g) on the right side, leaving us with the desired reaction (3):
C2H4(g) + H2(g)C2H6(g) ΔH° = -52.3 kJ + 169.4 kJ = 117.1 kJ
Learn more about standard enthalpy here :-
https://brainly.com/question/28303513
#SPJ11
IF X=3 AND Y=2,EVALUATE (XY) 2 and it's math not chemistry
Answers
Answer:
i really dont know but if you had answer choices it would be easier my guess is x^3 y^2
Explanation:
Which of the following statements is true?

Answers
the answer is the third one
Explanation:
it just is I think