the molar mass of vanadium is 50.99 what is its equivalent mass when it reacts with chlorine to form vcl5
Answers
To determine the equivalent mass of vanadium when it reacts with chlorine to form VCl5, we need to consider the molar mass of vanadium and the stoichiometry of the reaction.
Since there are five chlorine atoms reacting with one vanadium atom, we divide the molar mass of vanadium by 5 to find the equivalent mass.
Equivalent mass = Molar mass of vanadium / Number of chlorine atoms reacting with one vanadium atom
Equivalent mass = 50.99 g/mol / 5
Equivalent mass = 10.198 g/mol
To know more about that equivalent visit:
https://brainly.com/question/14672772
#SPJ11
Related Questions
As a professor Daniel must complete a research project each year in order to
stay on staff. Daniel is an engineer and has to deal with many complicated
mathematical calculations. Knowing this fact Daniel would most likely concern
himself with qualitative research,
Answers
Explanation:
Add our site sources in order to be advantage of knowledge
How does the nervous system work with the muscular system?
A: Receptors in muscles provide the brain with information about body position and movement.
B:Respiratory System and the Muscles of Inhalation and Exhalation.
C:It doesn't
D: Tendons connect the skeletal system to the muscular system by attaching muscle to bone.
Answers
Answer:
A. Receptors in muscles provide the brain with information about body position and movement.
Explanation:
What is the pH and final composition of the resulting solution if it contains 10-2 M of both NH4Cl and NaHS
Answers
The final composition of the resulting solution of 10^-2 M NH4Cl and NaHS will have a pH of 9.36.
We are given the concentration of NH4Cl and NaHS as 10^-2 M.The ammonium ion (NH4+) will undergo hydrolysis in water and form NH3 and H+.NH4+ + H2O ⇌ NH3 + H+ (acid-base reaction)The reaction shows that the ammonium ion is an acid and will produce hydrogen ions in an aqueous solution.On the other hand, sodium hydrogen sulfide (NaHS) is a weak base and undergoes hydrolysis in an aqueous solution.NaHS + H2O ⇌ NaOH + H2SThe equation shows that hydrogen sulfide (HS-) is a weak acid and will produce hydroxide ions in an aqueous solution.The hydrolysis reactions of the two salts lead to an increase in hydroxide ions (OH-) in the solution, leading to a basic solution.
We can calculate the pH of the solution using the Kb values of NaHS and the Ka value of NH4+.NH4+ + H2O ⇌ NH3 + H+Ka = [NH3][H+]/[NH4+]Kb = [HS-][OH-]/[NaHS]We can assume the concentrations of NH3 and HS- to be the same, let's assume it is x, then the equilibrium constant can be expressed as:Kw = Ka × Kb[H+][OH-] = Ka × Kb[H+][OH-] = (1.8 × 10^-5) × (1.2 × 10^-13) = 2.16 × 10^-18pH + pOH = 14pH + pOH = 14pH = 14 - pOHpOH = -log[OH-] = -log(1.47 × 10^-8) = 7.83pH = 14 - 7.83 = 6.17We can conclude that the pH of the resulting solution will be 9.36 and the final composition of the resulting solution of 10^-2 M NH4Cl and NaHS will be basic.
To know more about solution visit:
https://brainly.com/question/15757469
#SPJ11
What is your evidence? This can be in bulleted points.
can you help I don't now how to explain it.

Answers
as the temperature of a gas decreases is volume
Answers
Answer:
it's volume also decrease
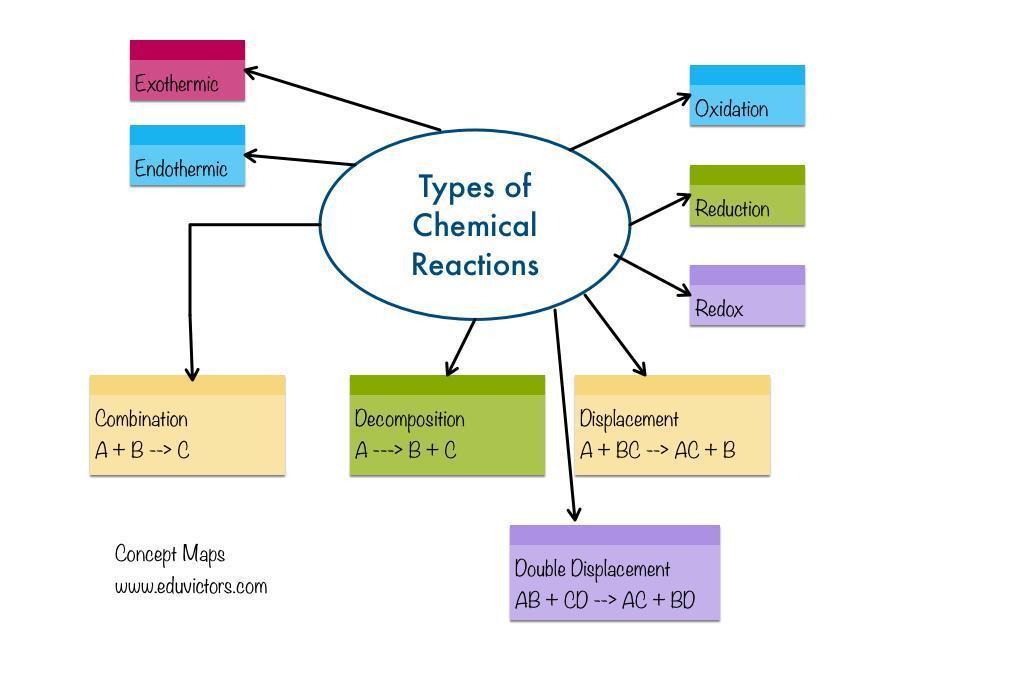
chemical change grade 10 mind map
Answers
Chemical change: A chemical reaction is the change of one chemical substance into another chemical substance. For instance: The rusting of iron, the curdling of milk, the digestion of food, breathing, etc.
What is a chemical reaction?
A chemical reaction results in a chemical change because a new material has entirely different properties from the original substance. In a chemical reaction, atoms rearrange themselves.Reactants are the chemicals that participate in a chemical reaction.Products are the new compounds created as a result of a chemical process. An illustration of a chemical reaction is burning magnesium in the air to produce magnesium oxide.2Mg(s) + O2(g) △→ 2MgO(s)The magnesium ribbon is cleaned with sandpaper before being burned in the air. This cleans the magnesium ribbon's surface of the basic magnesium carbonate protecting coating.Reactant: Materials that participate in a chemical reaction are referred to as reactants. Mg and O2, as an example.A product is a newly created substance that results from a chemical reaction. Example: MgO.A chemical reaction is the change of one chemical substance into another chemical substance.
To learn more about chemical reactions, refer to:
https://brainly.com/question/1222323
#SPJ9

Chemical change is the change chemical substance is transformed into another chemical substance.It is irreversible in nature , for example Reaction of medicine in body , milk to curd etc.
What is the difference between chemical and Physical change?1)Physical change temporary or reversible in nature but chemical change is irreversible in nature
2)In physical change there no new product is formed but in chemical change there formation of new product takes .
3) Physical change is change sin shape ,size or state for example freezing of water , melting of wax , and example of Chemical change are Burning of coal, digestion of food
to learn more about chemical change click here https://brainly.com/question/28089135
#SPJ9
What do you think is happening to the soda and air in the can?
Answers
The soda and air will bubble, increasing the chances of an explosion.
Carbonation in soda is made up of carbon dioxide or CO2 bubbles. Carbonated beverages are injected with this colorless and odorless gas under high pressure during manufacture until the liquid is supersaturated with gas. Individuals determined, at the discretion of the Federal Aviation Surgeon, to have a static or non-progressive disqualifying condition.
In soda ash, the dispersed phase is a gas and the dispersion medium is a liquid. Soda ash is an example of a gas that dissolves in a liquid. Shaking the can introduces many small air bubbles into the liquid, so the dissolved gas is more likely to combine with existing air bubbles and evaporate rather than form new air bubbles. By avoiding the difficult step of shaking and frothing the soda, the gas can escape faster creating more fizz.
Learn more about The soda and air here:-https://brainly.com/question/18187020
#SPJ1
6. XY₂ is the molecular formula of an ionic compound, where X is a metal! and also a representative element. Identify the groups of X and Y in the periodic table.
Answers
XY₂ is the molecular formula of an ionic compound where X is magnesium (Mg) and Y is chloride (Cl) and magnesium is metal of second group of the periodic table and represents the 2nd group and chloride belongs to 17th group.
What are ionic compound?Ionic compounds are those which completely dissociate into their constituent ions in water one is metal and other is non metal.
They complete their octave by sharing lone pair of electron and attend the positive and negative charge after dissociation are know as ionic compounds.
Therefore, the molecular formula of an ionic compound where X is magnesium (Mg) and Y is chloride (Cl) and magnesium is metal of second group of the periodic table and represents the 2nd group and chloride belongs to 17th group.
Learn more about ionic compound, here:
https://brainly.com/question/7655594
#SPJ1
A sample of gas has a volume of 5.0 L and a pressure of 2.92 atm. If the final volume is 5.7 L, what is the final pressure of the gas?
Answers
Answer: 2.56140351 atm
The equation we use is Boyle's law,
P1V1 = P2V2
where the numbers 1 and 2 represent the first and second conditions. All we have to do is rearrange the equation to solve for the volume.
To obtain the final pressure of the gas(P2), use the equation
P2 = \(\frac{P1V1}{V2}\)
so P2 = \(\frac{2.92 * 5}{5.7}\) = 2.56140351 atm.
The final pressure of the gas = 2.56140351 atm.
To learn more about pressure and volume,
https://brainly.com/question/15268286
if you have 3 dozen pennies how many ml of water would they displace
Answers
The mass of the displaced fluid can be expressed in terms of the density and its volume, m = ρV. The fluid displaced has a weight W = mg, where g is acceleration due to gravity. Therefore, the weight of the displaced fluid can be expressed as W = ρVg.
A motorcycle is moving at a constant speed of 35 km/h. How long (in hours) does it take the motorcycle to travel a distance of 62 km?
Answers
Answer:
Explanation:
Answer:
Hope this helps !
Explanation:

the oxidation number of a nitrogen atom in n₂o₃ is
Answers
A nitrogen atom in N2O3 has an oxidation number of +3.
The unknown nitrogen oxidation number can be given a variable (x) to ascertain its oxidation number. Since oxygen has an oxidation number of - 2 and there are three oxygen particles in N₂O₃, the complete negative charge from oxygen is (- 2) × 3 = - 6.
The total charge of a compound is equal to the sum of its oxidation numbers. Since the compound in question is neutral, the sum of the oxidation numbers must be zero in this instance.
2(N) + 3(O) = 0
2x + (-6) = 0
2x = 6
x = 3
To learn more about oxidation numbers:
https://brainly.com/question/4222605
What kind of organic compound is ethanoic acid?
Answers
Answer:
Acetic acid , systematically named ethanoic acid , is a colourless liquid organic compound with the chemical formula CH3COOH.
Explanation:
Write the balanced complete ionic equations and net ionic equations for the reactions that occur when each of the following solutions are mixed. (Type your answers using the format [NH4]+ for NH4+ or Ca3(PO4)2 for Ca3(PO4)2. Use the lowest possible coefficients.)(a) Cr2(SO4)3(aq) and (NH4)2CO3(aq)complete ionic equation:(aq) + CO32-(aq) + Cr3+(aq) + SO42-(aq) (s) + NH4+(aq) + (aq)net ionic equation:Cr3+(aq) + (aq) (s)(b) FeCl3(aq) and Ag2SO4(aq)complete ionic equation:(aq) + Cl-(aq) + Ag+(aq) + SO42-(aq) (s) + Fe3+(aq) + (aq)net ionic equation:Ag+(aq) + (aq) (s)(c) Al2(SO4)3(aq) and K3PO4(aq)complete ionic equation:(aq) + PO43-(aq) + Al3+(aq) + SO42-(aq) (s) + K+(aq) + (aq)net ionic equation:Al3+(aq) + (aq) (s)
Answers
(a) \(Cr_2(SO_4)_3\)(aq) and \((NH_4)_2CO_3\)(aq)
Complete ionic equation: \(Cr^{3+\)(aq) + \(3SO_{42}\)-(aq) + 2\(NH_4\)+(aq) + \(CO_3^{2-}\)(aq) → \(Cr_2(CO_3)_3\)(s) + 6\(NH^{4+}\)(aq) + 6\(SO_4^{2-}\)(aq)
Net ionic equation: \(Cr^{3+\)(aq) + 3 \(CO_3^{2-}\)(aq) → \(Cr_2(CO_3)_3\)(s)
(b) \(FeCl_3\)(aq) and\(Ag_2SO_4\)(aq)
Complete ionic equation: \(Fe^{3+\)(aq) + 3Cl-(aq) + 2Ag+(aq) + \(SO_4^{2-}\)(aq) → 2AgCl(s) + \(Fe^{3+\)(aq) + \(SO_4^{2-}\)(aq)
Net ionic equation: 2Ag+(aq) + 2Cl-(aq) → 2AgCl(s)
(c) \(Al_2(SO_4)_3\)(aq) and \(K_3PO_4\)(aq)
Complete ionic equation: \(2Al^{3+\)(aq) + 6\(SO_4^{2-}\)(aq) + 6K+(aq) + 2\(PO_4^{3-}\)(aq) → \(Al_2(PO_4)_3\)(s) + 6K+(aq) + 6\(SO_4^{2-}\)(aq)
Net ionic equation: \(2Al^{3+\)(aq) + 2\(PO_4^{3-}\)(aq) → \(Al_2(PO_4)_3\)(s)
These are examples of double displacement or precipitation reactions, where two solutions containing ionic compounds are mixed and an insoluble product (precipitate) is formed.
The complete ionic equation shows all the ions present in the solution before and after the reaction, while the net ionic equation only includes the ions that participate in the formation of the precipitate.
In each reaction, the cations and anions switch partners to form new compounds. In the complete ionic equation, each ion is shown as either aqueous (aq) or solid (s) based on whether it remains in solution or forms a solid precipitate.
In the net ionic equation, only the ions that form the solid product are included, and any spectator ions that do not participate in the reaction are removed.
To know more about "Solid" refer here:
https://brainly.com/question/5654381#
#SPJ11
25 cm³ of 0.1 mol/dm³ hydrochloric acid exactly neutralise 20 cm³ of aqueous sodium hydroxide.
The equation for this reaction is:
NaOH + HCI →→ NaCl + H₂O
What is the concentration of the sodium hydroxide solution?
Answers
The concept molarity is an important method which is used to calculate the concentration of a solution. Here molarity - volume relation is used to find out the concentration of the sodium hydroxide solution.
The term molarity is defined as the number of moles of the solute present per lite of the solution. It is represented as 'M' and it is expressed in the unit mol / L.
The equation connecting molarity and volume is:
M₁V₁ = M₂V₂
M₂ = M₁V₁ / V₂
0.1 × 25 / 20 = 0.125 cm³
To know more about molarity, visit;
https://brainly.com/question/15169023
#SPJ1
Fe2O3(s) + H2(g) = Fe(s) + H2O(l)
jenny does the experiment above and is able to produce 595 grams of iron. how many liters of hydrogen gas would she need to accomplish this?
Answers
Explanation:
\(molar \: mass \: of \: iron = 56 \: g \\ 56 \: g\: are \: weighed \: by \: 1 \: mole \: of \: iron \\ 595 \: g \: will \: be \: weighed \: by \: ( \frac{595}{56} ) \: moles \\ = 10.625 \: moles \: of \: iron \\ from \: equation : \\ 1 \: mole \: of \: iron \: is \: formed \: by \: 1 \: mole \: of \: hydrogen \\ 10.625 \: moles \: will \: be \: produced \: by \: (10.625 \times 1) \: moles \\ = 10.625 \: moles \: of \: hydrogen \\ at \: s.t.p : \\ 1 \: mole \: = \: 22.4 \: litres \\ 10.625 \: moles \: = (22.4 \times 10.625) \\ = 238 \: litres \\ \\ or \: at \: r.t.p : \\ 1 \: mole \: = 24 \: litres \\ 10.625 \: moles \: = \: (10.625 \times 24) \\ = 255 \: litres\)
¿por qué la teoría de Demócrito fue reemplazada por la teoría de Dalton y luego esta teoría fue reemplazada por por Rutherford.?
Answers
Answer:
Debido al descubrimiento de protones, neutrones y electrones.
Explicación:
La teoría de Demócrito fue reemplazada por la teoría de Dalton y luego la teoría de Dalton fue reemplazada por la de Rutherford debido al descubrimiento de protones, neutrones y electrones que están presentes dentro del átomo. Demócrito afirma que el átomo es una partícula indivisible de materia, pero el descubrimiento del protón, el neutrón y el electrón cambió este concepto de átomo, por lo que debido al mayor descubrimiento y estudio del átomo y sus partículas subatómicas, las teorías cambiaron con el paso del tiempo.
Whats the Net ionic and Total ionic equations of the decomposition of hydrogen peroxide when the catalyst is potassium iodide?
Answers
The total ionic equation is:
2H2O2 (aq) + 2KI (aq) → 2H2O (l) + O2 (g) + 2K+ (aq) + 2I- (aq)
What is the net ionic equation?
We have to know that the ionic equation would have to involve the ions that are found in the system. We know that the ions that we have in the system would comprise of the spectator ions and the ions that actually underwent a change.
The decomposition of hydrogen peroxide (H2O2) with potassium iodide (KI) occurs in the presence of a catalyst.
The net ionic equation of this reaction would then be;
H2O2 (aq) → H2O (l) + O2 (g)
Learn more about ionic equation:https://brainly.com/question/29299745
#SPJ1
Water forms according to the equation below: 2h2(g) o2(g) right arrow. 2h2o(g) delta.hrxn = -483.64 kj how much energy is released during the formation of 1 mol h2o(g)? kj
Answers
The amount of energy released during the formation of 1 mole of H2O(g) is -483.64 kJ divided by 2, which equals -241.82 kJ.
The given equation represents the formation of water (H2O) gas from hydrogen gas (H2) and oxygen gas (O2). The delta.hrxn value of -483.64 kJ indicates the heat energy released during this process.
To find out how much energy is released during the formation of 1 mole of H2O(g), we can refer to the stoichiometry of the balanced equation. The equation shows that 2 moles of H2(g) are required to form 2 moles of H2O(g).
Since the equation states that -483.64 kJ of energy is released during the formation of 2 moles of H2O(g), we can infer that half of this energy will be released when 1 mole of H2O(g) is formed.
Therefore, the amount of energy released during the formation of 1 mole of H2O(g) is -483.64 kJ divided by 2, which equals -241.82 kJ.
So, approximately -241.82 kJ of energy is released during the formation of 1 mole of H2O(g).
To know more about hydrogen gas visit:
https://brainly.com/question/32820779
#SPJ11
Is this a scientific model? Use complete sentences to explain why or why not. (5 points)
help quick

Answers
Answer:
yes
Explanation:
For each of the following pairs of compounds, choose which will elute faster in a TLC experiment (i. E. , which compound will have a larger Rf value). Explain what factors led to your choice. 6 pt a. Naphthalene or 1-Bromonaphthalene Choice Explanation: 1-Bromonaphthalene is more polar than Naphthalene. If polarity is higher, its Rf value will be less which means that molecule will travel less distance (lower Rf value) during a TLC experiment
Answers
In a TLC experiment, the compound with the larger Rf value will elute faster. In the case of naphthalene and 1-bromonaphthalene, the 1-bromonaphthalene will elute faster and have a larger Rf value.
This is because 1-bromonaphthalene is more polar than naphthalene. Polar compounds have a stronger attraction to the polar stationary phase (such as the silica gel in TLC plates) and will interact more with it, resulting in a lower Rf value.
Naphthalene, on the other hand, is less polar and will have a weaker interaction with the stationary phase, allowing it to travel further and have a higher Rf value.
The polarity of a compound is determined by the presence of functional groups or atoms that create an uneven distribution of charge or electronegativity. In this case, the bromine atom in 1-bromonaphthalene increases its polarity compared to naphthalene, leading to a stronger interaction with the stationary phase.
In summary, the 1-bromonaphthalene will elute faster in a TLC experiment and have a larger Rf value compared to naphthalene due to its higher polarity resulting from the presence of a bromine atom.
To know more about electronegativity refer to this:
https://brainly.com/question/3393418
#SPJ11
Compare the properties of the four states of matter in terms of particle arrangement, particle motion, shape, size, and compressibility.
Answers
Particle arrangement, shape, and size of the four states of matter generally decreases from solids to plasma, whereas particle motion and compressibility increases from solids to plasma.
What are the four states of matter?The four states of matter are:
solidliquidgasplasmaComparison of the properties of the four states of matter in terms of particle arrangement, particle motion, shape, size, and compressibility can be summarized as follows:
particle arrangement - orderliness decreases from solids to plasma particle motion - increases from solids to plasmashape - solids have definite shapes, whereas liquids, gases and plasma have no definite shapesize - solids have the largest size while plasma have the smallest sizecompressibility - solids are least compressible while plasma is most compressibleTherefore, the particle arrangement, shape, and size of the four states of matter generally decreases from solids to plasma, whereas particle motion and compressibility increases from solids to plasma.
Learn more about properties of states of matter at: https://brainly.com/question/3998772
#SPJ1
Acrylonitrile, C3H3N, is the starting material for
the production of a kind of synthetic fiber
acrylics) and can be made from propylene,
C3H6, by reaction with nitric oxide, NO, as
follows:
4 C3H6 (g) + 6 NO (g) → 4 C3H3N (s) + 6 H2O
(1) + N2 (g)
What is the limiting reagent if 168. 36 g of
C3H6 reacts with 180. 06 g of NO?
Answers
Acrylonitrile, C3H3N, is the starting material for the production of a kind of synthetic fiber acrylics) and can be made from propylene, the ratio of moles is less than the stoichiometric ratio of 4:6, \(C_3H_6\) is the limiting reagent.
To determine the limiting reagent, we need to compare the moles of each reactant and identify which one is present in the smallest amount. The limiting reagent is the one that will be completely consumed in the reaction, thereby determining the maximum amount of product that can be formed.
First, let's calculate the moles of each reactant using their molar masses:
Molar mass of \(C_3H_6\) (propylene): \(\(3 \times 12.01 + 6 \times 1.01 = 42.08 \, \text{g/mol}\)\)
Moles of \(C3H6\) = \(\(\frac{{168.36 \, \text{g}}}{{42.08 \, \text{g/mol}}} = 4.00 \, \text{mol}\)\)
Molar mass of NO (nitric oxide): \(14.01 + 16.00 = 30.01 \, \text{g/mol}\)
Moles of NO = \(\(\frac{{180.06 \, \text{g}}}{{30.01 \, \text{g/mol}}} = 6.00 \, \text{mol}\)\)
According to the balanced chemical equation, the stoichiometric ratio between \(C_3H_6\) and NO is 4:6. This means that for every 4 moles of \(C_3H_6\) 6 moles of NO are required.
To determine the limiting reagent, we compare the ratio of moles present. We have 4.00 moles of \(C3H6\)and 6.00 moles of NO. The ratio of moles for \(C3H6\) :NO is 4:6 or simplified to 2:3.
Since the ratio of moles is less than the stoichiometric ratio of 4:6, \(C_3H_6\) is the limiting reagent. This means that 4.00 moles of\(C_3H_6\) will completely react with 6.00 moles of NO, producing the maximum amount of product possible.
\(\[4 \, \text{C}_3\text{H}_6(g) + 6 \, \text{NO}(g) \rightarrow 4 \, \text{C}_3\text{H}_3\text{N}(s) + 6 \, \text{H}_2\text{O}(l) + \text{N}_2(g)\]\)
Learn more about limiting reagent here:
https://brainly.com/question/31171741
#SPJ11
Scientific knowledge has often been around for a long time, even thousands of years, and some of that knowledge is still correct today. For example, ancient astronomers figured out the length of a year and that length of a year is pretty much the same today in terms of astronomical 365 and a quarter days. Other things that have been around a long time have not necessarily held up. For thousands of years, astronomers believed that Earth was the center of the universe. They felt that everything else rotated around that and was finally disproven by Kepler and Galileo. And then again, we have things today that we believe that we may not believe in the future. For example, here is a little bit of scientific knowledge. Earth is the only planet to support life. Currently, that's pretty much what we believe, but there is a good chance in the future through advances in astronomy, optics, maybe we'll be able to see another planet with life, or through exploration of our moons and our solar system. Maybe there's life on some of the moons on Jupiter. Who knows? But again, scientific knowledge, it can last over time but we should always be questioning it.
Question 1
Is all scientific knowledge correct today?
A YesYes
B NoNo
Question 2
Should we always accept scientific knowledge?
A YesYes
B NoNo
Question 3
Advancements in _________ help us change the way we think about existing scientific knowledge.
A technologytechnology
B logiclogic
Answers
All scientific knowledge is correct today, it must be accepted , advancements in technology help us change the way we think about existing scientific knowledge.
Technology is the application of knowledge for achieving practical goals in a reproducible way. The word technology can also mean the products resulting from such efforts,including both tangible tools such as utensils or machines, and intangible ones such as software. Technology plays a critical role in science, engineering, and everyday life.
Technological advancements have led to significant changes in society. The earliest known technology is the stone tool, used during prehistoric times, followed by the control of fire, which contributed to the growth of the human brain and the development of language during the Ice Age.
Learn more about technology,here:
https://brainly.com/question/9171028
#SPJ1
What type of weathering occurs when acid rain changes rocks? A. erosion
B. deposition C. chemical weathering D. physical weathering
Answers
Answer:
c...................................
Calculate the volume of an object that has a mass of 20.00 g and a density of 2.45 g/cm^3.
Answers
Answer:
2450000 gram
Explanation:
v= m × ρ= 1 cubic meter × 2.45 gram/cubic centimeter= 1000000 cubic centimeter × 2.45 gram/cubic centimeter= 2450000 gram
Explanation:
v=m over D
mass=20.oog
Density=2.45g
20.00-2.45
ans is 17.46
Find the mass of benzene required to produce 3.50 L of carbon dioxide gas at ST in the following reaction.
2C6H6 + 1502- 12 CO, +6 H2O
Answers
The mass of benzene required to produce 3.50 L of carbon dioxide gas, CO₂ at STP in the reaction is 2.028 grams
How do i determine the mass of benzene required?First, we shall obtain the mole of carbon dioxide gas, CO₂ produced at STP. Details below:
At STP,
22.4 Liters = 1 mole of CO₂
Therefore,
3.5 liters = 3.5 / 22.4
3.5 liters = 0.156 mole of CO₂
Next, we shall obtain the mole of benzene, C₆H₆ required. Details below:
2C₆H₆ + 15O₂ -> 12CO₂ + 6H₂O
From the balanced equation above,
12 moles of CO₂ were obtained from 2 moles of C₆H₆
Therefore,
0.156 mole of CO₂ will be obtain from = (0.156 × 2) / 12 = 0.026 mole of C₆H₆
Finally, we shall obtain the mass of benzene, C₆H₆ required for the reaction. Details below:
Mole of C₆H₆ = 0.026 moleMolar mass of C₆H₆ = 78 g/molMass of C₆H₆ = ?Mass = Mole × molar mass
Mass of C₆H₆ = 0.026 × 78
Mass of C₆H₆ = 2.028 grams
Thus, the mass of benzene, C₆H₆ required is 2.028 grams
Learn more about mass needed:
https://brainly.com/question/29263739
#SPJ1
what reaction is used for anabolism? group of answer choices hydrolysis catabolic dehydration hydration
Answers
The reaction is used for the anabolism is the dehydration. that means the loss of the water molecules.
The water is the universal solvent. water is the chemically reactive compound and the number of the biochemical reactions takes place. The hydrolysis is the reaction in which use water molecule break the bonds in between the large compounds. the hydrolysis reaction is used in the catabolism. such as the depolymerization of the protein molecules.
In the condensation reaction , the two molecules joined to form the large molecule. this reaction will leads to the water molecule loss. this is called dehydration reaction. the dehydration reaction used in the anabolism reaction or anabolic reaction.
To learn more about anabolic here
https://brainly.com/question/14932822
#SPJ4
PLEASE ANSWER ALL I WILL GIVE BRAINLIEST PLEASE!!

Answers
how many different bases of DNA
Answers
Answer:
four
Explanation:
adenine, thymine, guanine, and cytosine