the mass spectrum for an unknown element is shown above. according to the information in the spectrum, the atomic mass of the unknown element is closest to 90amu 90 a m u 91amu 91 a m u 93amu 93 a m u 94amu
Answers
Because 1s electrons are most attracted to the nucleus, peak X results.
For A(27.977 u)
Mass number = 27.977
Abundance = 78.21%
For B (28.976 u)
Mass number = 28.976
Abundance = 1.346%
For C(29.974 u)
Mass number = 29.974
Abundance = 20.444%
Averages atomic mass
= (Mass of A x abundance of A)/100 + (Mass of B x abundance of B)/100 + (Mass of C x abundance of C)/100
= (27.977x78.21)/100 + (28.976x1.346)/100 + (29.974x20.444)/100
= 21.8808117 + 0.39001696 + 6.12788456
= 28.40
What is a nucleus?In terms of genomics, a nucleus is the organelle within a cell that is membrane-enclosed and houses the chromosomes. The nuclear membrane has a variety of pores that enable the selective passage of specific molecules (such proteins and nucleic acids) into and out of the nucleus.
The nucleus carries the genes, structures that hold the genetic information, and controls and regulates the functions of the cell (such as growth and metabolism).Inside the nucleus, tiny structures known as nucleoli are frequently seen. The gel-like matrix in which the nuclear components are suspended is known as the nucleoplasm.
To learn more about nucleus from the given link:
brainly.com/question/23366064
#SPJ4
Related Questions
Arrange the events of succession after a volcano erupted in their proper order
Answers
Answer:
pioneer species, soil formation, plant growth
Explanation:
The events of succession after a volcano erupted in the proper order include pioneer species being visible. The pioneer species such as algae and Moss are usually found on rocks and dead matter.
After sometime the soil starts to form through weathering activities which then gives rise to and supports the growing of plant species.
What is the absolute smallest thing we can break all matter down into without loosing it's property?
Answers
Answer:
Protons and neutrons can be further broken down: they're both made up of things called “quarks.” As far as we can tell, quarks can't be broken down into smaller components, making them the smallest things we know of.
Explanation:
pretend you have an container of salt and a container of salt and a container of trail mix , how could `you physically separate the sodium and the cholorine from the salt ? how could you seperate the pieces in the trail mix
Answers
Pretend you have an container of salt and a container of salt and a container of trail mix , separate the sodium and the chlorine from the salt is trail mix and sodium and chlorine from the electrolysis.
We known that the mixture is the compound in which there are two or more chemical compound are present. The trail mix is the heterogenous mixture. A heterogenous mixture is the mixture of uneven distribution of the components in the mixture. The given container a salt and a trial mixture. the trial mixture is the mixture of peanuts and the other seeds. we can physically separate the mixture of salt and trial mixture by Handpicking method. And we can separated the sodium and chlorine by the process of electrolysis.
Pretend you have an container of salt and a container of salt and a container of trail mix , separate the sodium and the chlorine from the salt is sodium and chlorine from the electrolysis.
To known more about separate mixture here
https://brainly.com/question/13619907
#SPJ1
english teacher of the 1700s who stated that matter is made of atoms that cannot be divided and that atoms combine in specific ratios.
true/false
Answers
Answer: False
Explanation: Flase Old John Dalton (1776-1894)
I hope this is helpful I know it is not much but this is a false answer
If 12.3 mol HCl are produced in this reaction, how many grams of sodium sulfate are produced?

Answers
ANSWER
The mass of Na2SO4 is 874g
EXPLANATION
Given that;
The number of moles of HCl is 12.3 mol
Follow the steps below to find the mass of sodium sulfate produced
Step 1; Write a balanced equation for the reaction
\(\text{ 2NaCl + H}_2SO_4\text{ }\rightarrow\text{ 2HCl + Na}_2SO_4\)In the reaction above, 2 moles of NaCl react with 1 mole of H2SO4 to give 2 moles of HCl and 1 mole of Na2SO4
Let the number of moles of Na2SO4 be x
\(\begin{gathered} \text{ 2 moles HCl }\rightarrow\text{ 1 mole Na}_2SO_4 \\ \text{ 12.3 moles HCl }\rightarrow\text{ x moles Na}_2SO_4 \\ \text{ Cross multiply} \\ \text{ 2 moles HCl }\times\text{ x moles Na}_2SO_4\text{ }=\text{ 1 mole Na}_2SO_4\text{ }\times\text{ 12.3 mole HCl} \\ \text{ Isolate x} \\ \text{ }\times\text{ }=\text{ }\frac{1\text{ mole Na}_2SO_4\times12.3mol\cancel{HCl}}{2moles\cancel{HCl}} \\ \text{ } \\ \text{ x = }\frac{1\text{ }\times\text{ 12.3}}{2} \\ \text{ x = 6.15 moles} \end{gathered}\)The number of moles of Na2SO4 is 6.15 moles
Step 3; Find the mass of Na2SO4 using the below formula
\(\text{ mole = }\frac{\text{ mass}}{\text{ molar mass}}\)Recall, that the molar mass of Na2SO4 is 142.04 g/mol
\(\begin{gathered} \text{ mass = mole }\times\text{ molar mass} \\ \text{ mass = 6.15 }\times\text{ 142.04} \\ \text{ mass = 873.546} \\ \text{ mass = 874g Na}_2SO_4 \end{gathered}\)Therefore, the mass of Na2SO4 is 874g
URGENT!! 20 Points Please Help!! WIll mark brainliest!! My assignment is due in 10 minutes!!
3.) Make the following conversions:
a.) 157cs to seconds
b.) 42.7L to milliliters
c.) 261nm to millimeters
d.) 0.065km to decimeters
e.) 642cg to kilograms
f.) 8.25 × \(10^{2}\)cg to nanograms
Answers
Answer:
a: 157cs = 1.57s
b: 42.7L = 42,700mL
c: 261nm = 0.000261mm
d: 0.0065km = 65dm
e: 642cg = 0.0064km
f: 8.25 x 10^2cg = 8,250,000,000ng
Answer:
a) 1.57
b) 42,700
c) 0.000261
d) 650
e) 0.00642
cant figure out f
Explanation:
When fertilization occurs one half of the DNA comes from the __________
and one half from the
_________
Answers
Answer:
Father - Mother
Sperm - Egg and those fertilized are called a Zygote
Explanation:
Answer:
one half from the father and the other half from the mother
Explanation:
hope it helps
I WILL MARK BRAINLY WHOLE SLIDE


Answers
intense heat waves and droughts
•FAMINE
•FOREST FIRES
powerful rain and snow STORMS
•FLOODING?? (could possibly be hurricane)
•Water borne and insect borne DISEASE
Increased HURRICANE (could possibly be flooding) and tornadoes DESTRUCTION of homes, ecosystems, and death.
I tried lol good luck though.
Your next-door neighbor has a little girl who is 3 ft, 5 inches tall. What is the little girl’s height measured in inches? in decimeters?
Answers
Answer:
41 inches
Explanation:
There is 12 inches in 1 foot so times 12 and 3. Thats 36 so now do 36 + 5 that =41.
What is the name of this compound?
C6H6-
C=O-
H

Answers
Answer: Benzaldahyde
Explanation: the C₆H₅- represents the substituted benzene ring and the
CHO should represent the functional group of aldehyde
Answer:
The name of the compound is benzaldehyde
Explain how the differences in valence electrons between metals and nonmetals lead to differences in charge and the giving or taking of electrons, ion formation
Answers
The differences in valence electrons between metals and nonmetals play a crucial role in determining the charge and the giving or taking of electrons during ion formation.
Valence electrons are the outermost electrons in an atom that participate in chemical reactions. Metals typically have few valence electrons, while nonmetals tend to have more valence electrons. This disparity in electron configuration creates an imbalance in electron distribution between the two groups. Metals, which have fewer valence electrons, tend to lose these electrons to achieve a stable electron configuration similar to the nearest noble gas. By losing valence electrons, metals form positively charged ions known as cations. The loss of electrons creates a deficiency of negative charges, resulting in a net positive charge on the ion. Nonmetals, on the other hand, have a greater affinity for electrons due to their higher valence electron count. They tend to gain electrons from other atoms to achieve a stable electron configuration resembling the nearest noble gas. By gaining electrons, nonmetals form negatively charged ions called anions. The addition of electrons results in an excess of negative charges, leading to a net negative charge on the ion. The transfer of electrons between metals and nonmetals during ion formation is driven by the desire to achieve a more stable electron configuration. The electrostatic attraction between the oppositely charged ions (cations and anions) results in the formation of ionic compounds. In summary, the differences in valence electrons between metals and nonmetals dictate the charge and the giving or taking of electrons during ion formation. Metals lose electrons to form positive cations, while nonmetals gain electrons to form negative anions. This transfer of electrons enables the formation of ionic compounds and helps achieve a more stable electron configuration for both metal and nonmetal atoms.
Learn more about Valence electrons here:
https://brainly.com/question/31264554
#SPJ11
what is the original source of electrons for psii?
Answers
Answer: In the photosystem II (PSII) reaction center, energy from sunlight is used to extract electrons from water. Explantion: The electron transport chain moves protons across the thylakoid membrane into the lumen.
The literature value for the melting point of your product was 154-155 °C. Below is the data for 4 students, which student had the purest crystals AND the correct product. Student A: melting point range of 141-147 °C Student B: melting point range of 158-159 °C Student C: melting point range of 145-155 °C Student D: melting point range of 151-152 °C B с D A
Answers
Therefore, Student C had the purest crystals and the correct product
Melting point determination is a useful technique for checking the purity and identity of the crystalline solid. The melting point of a compound is the temperature range where the solid changes to a liquid. The literature value for the melting point of a product is an important property to know, and it can be compared to the values obtained experimentally.
The melting point of the compound is an important factor in determining its purity. A pure compound has a sharp melting point, whereas an impure compound has a broad melting range that is lower than the pure melting point.
The student with the purest crystals and the correct product was Student C.
Student A's melting point range is too low, which indicates that the compound is impure.
The melting point range of Student B is too high, which indicates that the compound is impure.
The melting point range of Student D is close to the literature value, but it is still too broad, which indicates that the compound is impure.
Student C's melting point range is close to the literature value, and it is relatively sharp, which indicates that the compound is pure.
to know more about melting point visit:
https://brainly.com/question/31109629
#SPJ11
Answer the following questions
1. Why H₂O is a liquid and H₂S is a gas at room temperature
2. Arrange the following in increasing boiling point and explain. He, Br₂, NaCl
3. State the type of bonding between all the atoms and species in NH4Cl
4. Which do you expect to form the strongest ionic bond? NaCl or Nal
5. What effect does hybridization have on bonds?
Answers
Hybridization helps bonds to have sufficient energy for the interaction to take place.
How is boiling point related to the intermolecular bonding?Water contains oxygen which is more electronegative that sulfur and this leads to a greater intermolecular hydrogen bonding and molecular association
The arrangement of the molecules in the order of increasing boiling point is;He < Br₂ < NaCl. This is because the ionic bond in the NaCl makes it to have the greatest molecular association compared to the other two held together by weal dispersion forces.
The bonding types in NH4Cl are;
Ionic
Covalent
Coordinate covalent
NaCl would have stronger ionic bonding that NaI due to the greater electronegativity of the chlorine atom.
Learn more about boiling point:https://brainly.com/question/2153588
#SPJ1
If a 50kg object is moving at a velocity of 1 m/s how much kinetic energy does the object have
Answers
Answer:
25 JExplanation:
The kinetic energy of an object can be found by using the formula
\(k = \frac{1}{2} m {v}^{2} \\ \)
m is the mass
v is the velocity
From the question we have
\(k = \frac{1}{2} \times 50 \times {1}^{2} \\ = 25 \times 1 = 25\)
We have the final answer as
25 JHope this helps you
Calculate the shortest wavelength of the electromagnetic radiation emitted by the hydrogen atom in undergoing a transition from the n = 6 level.
Answers
Answer:
\(\lambda = 9.376*10^{-8} m\)
Explanation:
From the question we are told that:
\(n=6level\)
Generally the equation for Energy is mathematically given by
\(E=\frac{hc}{\lambda}\)
Since
Energy difference will be maximum when electron return to ground state
And Shortest wavelength is emitted when there is largest energy difference
Therefore
\(1/\lambda = R* (\frac{1}{nf^2} - \frac{1}{ni^2})\)
Where
\(R=Rydberg\ constant\)
\(R = 1.097*10^7\)
Therefore
\(1/\lambda = 1.097*10^7* ((\frac{1}{1^2} - \frac{1}{6^2})\)
\(\lambda = 9.376*10^{-8} m\)
Scientists can identify an element by looking at the structure of a single
Answers
Answer:enzyme
Did i help you if so leave a thanks if not...than i will better try to explain the anwser or look into it a bit more to get the correct one.
Calculate the mole fraction of each component in a solution of 42 g CH3OH, 35 g of chloroform CHCl3, and 50 g C3H7OH. Show your work.
Answers
Given the masses of the three components, we can use the molar masses to calculate the number of moles of each:
CH3OH: 42 g / 32.04 g/mol = 1.312 moles
CHCl3: 35 g / 119.38 g/mol = 0.293 moles
C3H7OH: 50 g / 60.1 g/mol = 0.832 moles
The total number of moles of the solution is the sum of the moles of the three components:
1.312 moles + 0.293 moles + 0.832 moles = 2.437 moles
Now we can calculate the mole fraction of each component by dividing the number of moles of each component by the total number of moles:
Mole fraction of CH3OH = 1.312 moles / 2.437 moles = 0.538
Mole fraction of CHCl3 = 0.293 moles / 2.437 moles = 0.120
Mole fraction of C3H7OH = 0.832 moles / 2.437 moles = 0.342
Therefore, the mole fraction of CH3OH is 0.538, the mole fraction of CHCl3 is 0.120, and the mole fraction of C3H7OH is 0.342.
Mark the statements which are correct. (Select all that apply. )
1 g = 10^3 mg
10^-3 g = 10^12 ng
1 s = 10^6 μs
1 km = 10^5 mm
1 s = 10^3 ms
Answers
All statements given in the question are incorrect except for 1 statement. The correct statement is:1 s = 10^3 ms.
In the question, we have been provided with 5 statements. We are asked to select all the correct statements from those 5 statements. Given below are for each statement:1 g = 10^3 mg:This is incorrect. 1 g is equal to 1000 mg.10^-3 g = 10^12 ng:This is incorrect. 10^-3 g is equal to 1 mg.1 km = 10^5 mm:This is incorrect. 1 km is equal to 1,000,000 mm.1 s = 10^6 μs:This is incorrect. 1 s is equal to 1,000,000 μs.1 s = 10^3 ms:This is correct. 1 s is equal to 1000 ms.Therefore, the main answer to this question is that only 1 statement is correct, which is:1 s = 10^3 ms.
Metric units are based on the power of ten. The base units of the International System of Units (SI) are the meter, kilogram, second, kelvin, ampere, mole, and candela. All other metric units can be derived from these basic units.The first unit in each conversion is in grams, seconds, or kilometers. The metric units for millimeters, microseconds, and nanograms are derived from these basic units. One gram is equal to 1000 milligrams (mg), 1 second is equal to 1000 milliseconds (ms), and 1 kilometer is equal to 1000000 millimeters (mm). 10^-3 g is equal to 1 milligram (mg), 10^6 μs is equal to 1 second (s), and 10^12 ng is equal to 1 gram (g).
To know more about except visit:
https://brainly.com/question/14400269
#SPJ11
Synthetic materials are not found in nature and therefore they are typically developed in laboratories.
True
False
Answers
Answer: True
Explanation: Synthetic materials are not found in nature and can be created in labs using chemicals and compounds.
When piece of very hot metal releases energy into a cup of water, if the temperature of metal drops
by 426.7°C, and the final temperature of the metal is 31.2°C, what was the initial temperature of
the metal?
Answers
Answer: The answer is 457.9
Explanation: Realistically all you have to do is add both of the temperatures and then you can figure out the original heat. Since the heat drops 426.7 degrees and it comes to 31.2 degrees its 426.7 + 31.2 = 457.9.
8. Calculate the pH of a solution if the [OH-] concentration is 0.015 M.
A) 1.82
B) 8.82
C) 12.18
D) 4.32
Answers
Answer:
1.82
Explanation:
pH is given by the equation
There are four types of charges present in Oxide. Draw a graph
and describe how each feature appears in C-V.
Answers
Oxides contain four types of charges: fixed charges (Qf), trapped charges (Qt), interface charges (Qit), and mobile ions (Qm).C-V graphs are used to assess the electrical characteristics of a dielectric interface. C is the capacitance of the oxide layer, and V is the applied voltage on the metal electrode that forms the oxide layer.
As the capacitance of the oxide layer changes with the applied voltage, the C-V graph shows the capacitance change. The graph below shows how each feature appears in a C-V graph.
[Blank]Fixed charge (Qf)Fixed charges are immobile, so they can only interact with the applied voltage via their electrostatic effect. As a result, when the applied voltage is greater than a specific threshold voltage (VT), the fixed charges create a dip in the C-V graph.
[Blank]Mobile ions (Qm)Mobile ions are also present in the oxide layer, and they can move in response to an electrical field. The mobile ions influence the electrostatic potential in the oxide layer, which alters the capacitance. Because of this influence, the C-V graph has a tiny dip before the hump known as the tail.
To know more about electrical visit:
https://brainly.com/question/31173598
#SPJ11
how do I balance this?

Answers
Answer:
4NaHCO3---->2Na2CO3+2CO2+2H2O
Calculate the mass of chromium that can be formed from 1.25 kg of chromium oxide
Answers
The balanced chemical equation for the reaction is:
2 Cr2O3 + 3 Al -> 4 Cr + 3 Al2O3
This equation tells us that two moles of chromium oxide (Cr2O3) react with three moles of aluminum (Al) to produce four moles of chromium (Cr) and three moles of aluminum oxide (Al2O3).
To calculate the mass of chromium that can be formed from 1.25 kg of chromium oxide, we need to use stoichiometry.
First, we need to determine the number of moles of chromium oxide in 1.25 kg of chromium oxide. The molar mass of Cr2O3 is 152 g/mol, so 1.25 kg (or 1250 g) of Cr2O3 is equal to:
1250 g / 152 g/mol = 8.22 mol
According to the balanced chemical equation, 2 moles of Cr2O3 produce 4 moles of Cr. Therefore, 8.22 moles of Cr2O3 will produce:
4/2 x 8.22 mol = 16.44 mol of Cr
Finally, we need to convert the number of moles of chromium produced to mass. The molar mass of Cr is 52 g/mol, so:
16.44 mol x 52 g/mol = 855.36 g
Therefore, the mass of chromium that can be formed from 1.25 kg of chromium oxide is approximately 855.36 grams, or 0.85536 kg.
Which of the following is a chemical property of a fossil fuel?
A. ability to burn
B. dark color
C. phase of matter
D. density
Answers
A superficial dermal erythema that is classified as an exanthem is more commonly known as a(n) __________
Answers
Answer:
ITS CALLED RASH
Explanation:
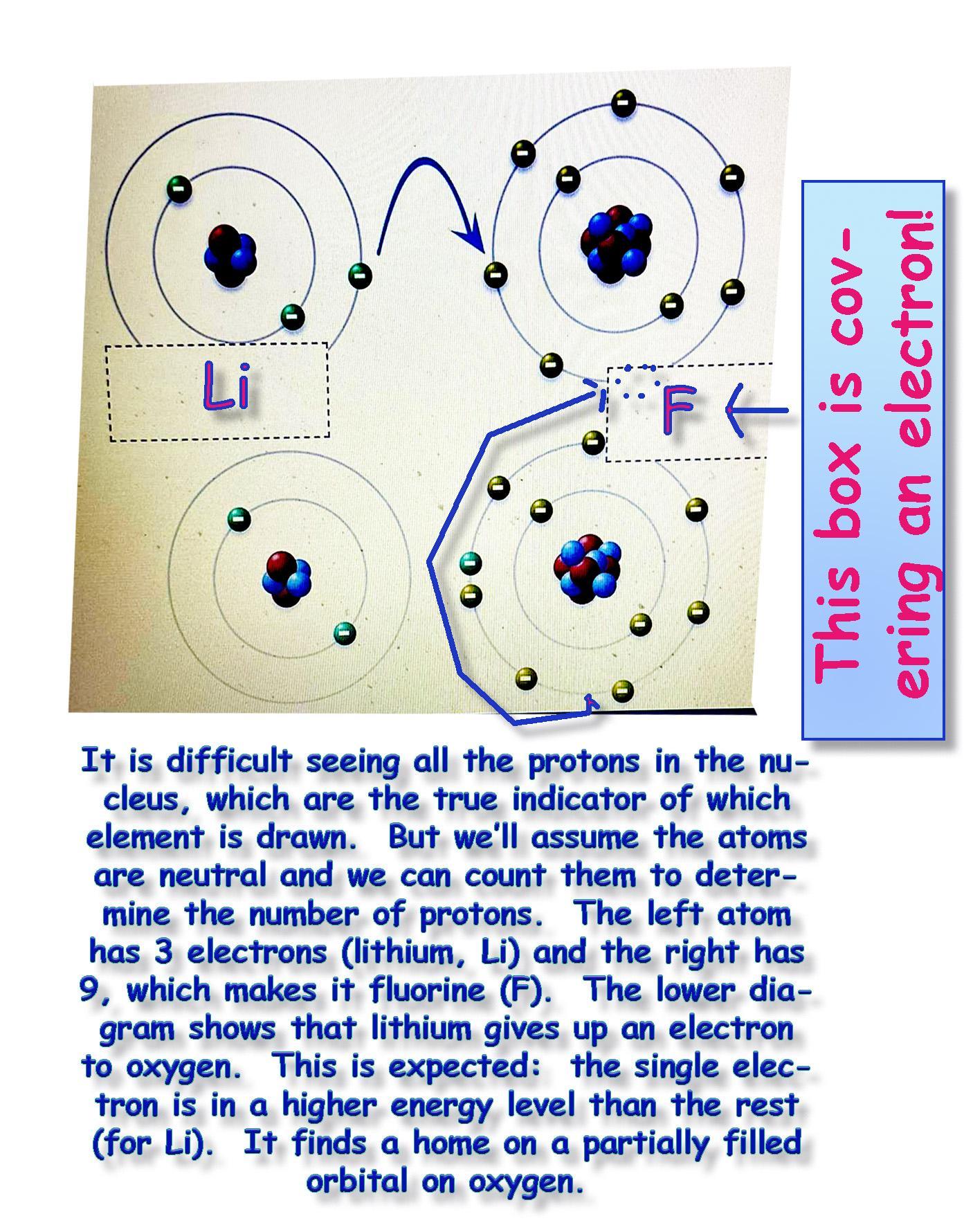
Label the diagram of ionic bonds with the name of the 2 elements involved
the options are
Lithium, fluorine, sodium and chlorine
And it has a picture of the periodic table above Brainly just let's me use one picture at a time and
that was the most important
I took 3 pictures the first one was the periodic table, the second one is shown and the third were just the options listed above ^

Answers
Answer:
Lithium (Li) and Fluorine (F)
Explanation:
Assume the atom is neutral and count the electrons. That will also be the number of protons (i.e., the atomic number).
See attached worksheet.
what does le chateliter's principle state
Answers
calculate the mass of the zinc that reacts with 4.11 g of hydrochloric acid to form 9.1 g of zinc chloride and 3.97 g of hydrogen acid
Answers
4.11 + x = 9.1 + 3.97
x = 8.87 grams
8.87 grams of zinc required