the ____ is the attraction of the electrons of one atom to the protons of the other atom leading a bond.
Answers
Related Questions
why is it important to perform the fractional distillation slowly? what happens if a fractional distillation is done very quickly by rapidly raising the temperature of the heat source?
Answers
To accomplish a satisfactory separation, a slow, even distillation is required since only under these circumstances can vapor and liquid in the distillation apparatus be in balance with one another.
What justifies using a slow fractional distillation process? What would occur if it was completed too soon?The best equilibration and heat transport can essentially be achieved through slow, steady distillation. If you heat the mixture too quickly, the vapors could not condense as quickly as you'd want, wasting some of the column. Packaging supplies are also essential. Packing materials with a large surface area give surfaces on which condensation can happen.
How does too-rapid distillation affect the process?Additionally, bumping may happen if the distillation proceeds too quickly. -The target liquid's vapors condense too soon and fall back into column.
To know more about fractional distillation visit:-
https://brainly.com/question/29037176
#SPJ4
Plzzzzzzzzz help whole slide

Answers
Answer: in order, cellular respiration, carbon dioxide, agricultural, methane, digestion, volcanic activity, eruption
Explanation:
its hard to explain why these things are thing you will have to memorize at first and then as you get later into science, you will learn about chlorophyll and mitochondria, and all sorts of fun things!
In your OWN words, what is the difference between a solute, solvent, and solution?
Answers
Answer:A solution is a homogeneous mixture consisting of a solute dissolved into a solvent . The solute is the substance that is being dissolved, while the solvent is the dissolving medium.
Explanation:A solution is a homogeneous mixture consisting of a solute dissolved into a solvent . The solute is the substance that is being dissolved, while the solvent is the dissolving medium.
PLEASEEEEE CAN SOMEONE HELP MEEE??? (iI have TWO questions PLSPLSPLSPSLPLSPLSPLSP)
3.) What happened to Patroclus at the end of yesterday's reading?
He won the battle.
He went to Mt. Olympus to talk to Zeus.
He died.
-------------------------------------------------------------------------------------------------------
4.Who was Agamemnon trying to persuade to come fight with him again?
Patroclus
Achilles
Odysseus
Hector
What is the value for AG at 300 K if AH = 27 kJ/mol and AS = 0.09 kJ/(mol-K)?
O
A. AG = 54 kJ/mol
O
B. AG=-18 kJ/mol
O
C. AG= 0 kJ/mol
O
D. AG = 27 kJ/mol
Answers
Answer:
0 kJ/mol
Explanation:
Step 1: Given data
Enthalpy change (ΔH): 27 kJ/molEntropy change (ΔS): 0.09 kJ/mol.KAbsolute temperature (T): 300 KStep 2: Calculate the Gibbs free energy change (ΔG)
We can calculate the Gibbs free energy change using the following expression.
ΔG = ΔH - T × ΔS
ΔG = 27 kJ/mol - 300 K × 0.09 kJ/mol.K = 0 kJ/mol
Since ΔG = 0 kJ/mol, the process in not spontaneous nor non-spontaneous.
When the temperature of the water was 10°C the waterweed did not produce bubbles.
Suzi increased the temperature of the water in the water-bath to 20°C. The waterweed started to produce bubbles.
She waited two minutes before starting to count the bubbles.
Explain why she waited for two minutes before she started to count the bubbles.
Science
Answers
Explanation:
she could waited for the saturated vapour pressure to equal the prevailing atmospheric pressure
ergo boiling to take place seeing as she had gotten the first indication that boiling had started
How are stoichiometric calculations performed for redox reactions?
Answers
Answer:
(b) Both Assertion and Reason are correct but Reason is not the correct explanation for Assertion
Explanation:
According to the law of conservation of mass, matter can neither be created nor be destroyed. However, it can be transformed from one form into another. During chemical reactions, atoms or ions are exchanged between reactants to form products. Thus, all the stoichiometric calculations are based on law of conservation of mass.
During redox reactions, one species is oxidized and other species is reduced. This involves electron transfer.
A cylinder contains 28.5 L of oxygen gas at a pressure of 1.8 atm and a temperature of 298 K. How much gas (in moles) is in the cylinder
Answers
To solve this problem, we can use the Ideal Gas Law equation: PV = nRT. Where P is pressure, V is volume, n is the number of moles of gas, R is the gas constant, and T is temperature. First, we need to convert the given volume of 28.5 L to liters per mole (L/mol) by dividing by the molar volume of an ideal gas at standard temperature and pressure (STP), which is 22.4 L/mol.
Given:
P = 1.8 atm
V = 28.5 L (convert to liters if needed)
T = 298 K
R = 0.0821 L·atm/mol·K (ideal gas constant)
Step 1: Rearrange the Ideal Gas Law equation to solve for n: n = PV/RT
Step 2: Plug the given values into the equation: n = (1.8 atm × 28.5 L) / (0.0821 L·atm/mol·K × 298 K)
Step 3: Calculate the number of moles: n ≈ 2.18 moles
Therefore, there are approximately 2.18 moles of oxygen gas in the cylinder.
Learn more about volume here : brainly.com/question/1578538
#SPJ11
In the reaction of nitrogen gas with oxygen gas to produce nitrogen oxide, what is the effect of adding more oxygen gas to the initial reaction mixture
Answers
The Equilibrium would shift to produce more NO
The reaction is;
N₂(g) + O₂(g) ⇆ 2NO(g)
When a reaction is at equilibrium then the forward reaction rate will be equivalent to the reverse reaction rate. Additionally, the concentration of the reactants and products are the same.
Additional reactants favor the formation of more products while additional products favor the formation of more reactants.
For example, when more oxygen is added then more Nitrogen (II) oxide will be formed.
Oxygen is a reactant and when increased it favors forward reaction which leads to the formation of more NO which is the product.
What is Equilibrium reaction?Chemical equilibrium is the condition in which both reactants and products are present in concentrations that have no further tendency to change with time, resulting in no observable change in the system's properties. If the same conditions are maintained, it will be seen that 87 percent of ammonia decomposes to form nitrogen and hydrogen. Therefore, it is an example of a reversible equilibrium reaction.
To learn more about equilibrium reaction from the given link
https://brainly.com/question/18849238
#SPJ4
Which of the following is a TRUE statement about the polymer shown below? [SELECT ALL THAT APPLY.] A) At least one of the side chains shown can form hydrophobic interactions. B) All of the side chains in the amino acids of this peptide are identical. C) There are three peptide bonds in this molecule. D) The primary structure of this protein is shown in the diagram.
Answers
The correct statements based on the given polymer structure are:
A) At least one of the side chains shown can form hydrophobic interactions.
C) There are three peptide bonds in this molecule.
A) At least one of the side chains shown can form hydrophobic interactions.
Looking at the side chains in the polymer, we see the presence of a methyl group (-CH3) attached to a carbon atom. Methyl groups are typically nonpolar and hydrophobic in nature. Therefore, it can be concluded that at least one of the side chains shown can form hydrophobic interactions.
B) All of the side chains in the amino acids of this peptide are identical.
Examining the side chains in the polymer, we see different groups attached to the carbon atoms, including -SH, -CH2COOH, and -CH(CH3)2. These groups are distinct and not identical. Therefore, the statement that all of the side chains in the amino acids of this peptide are identical is false.
C) There are three peptide bonds in this molecule.
A peptide bond is formed between the carboxyl group (-COOH) of one amino acid and the amino group (-NH-) of another amino acid. By counting the number of amide bonds, we can determine the number of peptide bonds. In the given polymer structure, we observe four amide bonds, indicating that there are three peptide bonds.
D) The primary structure of this protein is shown in the diagram.
The primary structure of a protein refers to the linear sequence of amino acids. The given polymer structure does not provide the specific sequence of amino acids. Therefore, we cannot determine the primary structure of the protein from the diagram.
Therefore, the correct statements based on the given polymer structure are:
A) At least one of the side chains shown can form hydrophobic interactions.
C) There are three peptide bonds in this molecule.
Learn more about Peptide bonds from the link given below.
https://brainly.com/question/32355776
#SPJ4
You read a primary source and a secondary source that discuss the same
experiment. There is a difference in the conclusions made by these two
sources. Which should you trust more, and why?
A. The primary source, because it is firsthand information
B. The secondary source, because it is easier to read
C. The primary source, because it is more confusing
D. The secondary source, because it was in a newspaper
Answers
Giving BRAINLIEST, please HELP!
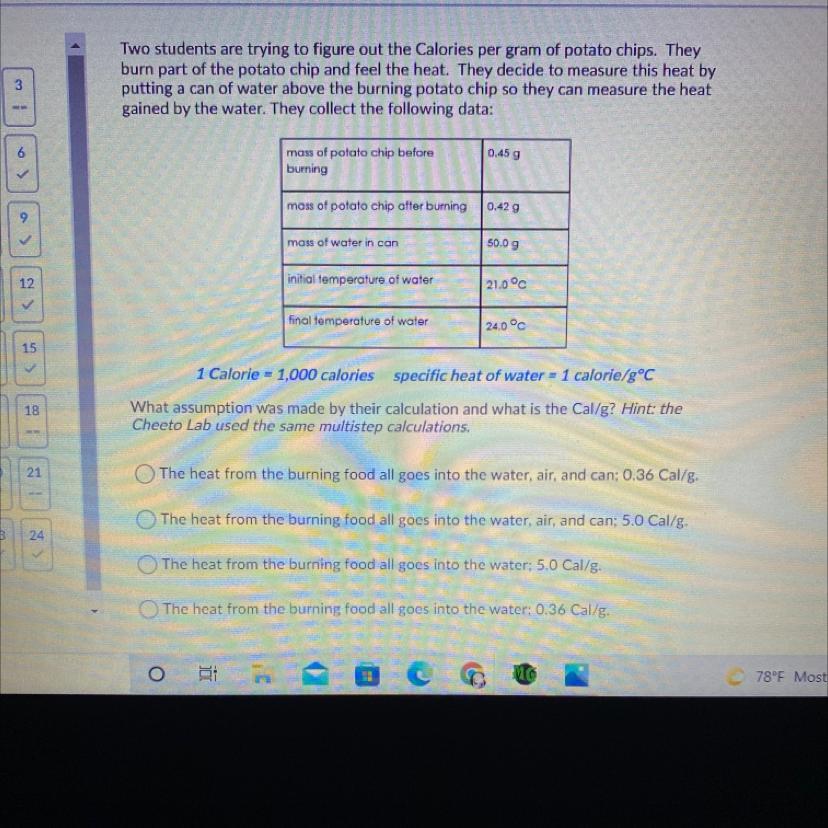
Answers
Answer:
Third choice see below
Explanation:
Weight change of potato chip .45 - .42 = .03 gm
Assume all of the heat from burning the chip goes into the water (and none goes into the air or heating the can)
24 degrees - 21 degrees = 3 degrees C temp change
spec heat water = 1 cal / g -cal
50 gm * 3 degrees * spec heat water = 150 calories
150 calories = 150/1000 Calories
Now for the potato chip
150/1000 Calories / .03 gm = 5 Cal/gm
The theoretical yield and the percent yield are calculated shown below. Did you perform the calculations correctly?

Answers
Answer:
\(56 \times { \frac{01514344}{?} }^{2} 5566648443hffii51 \\ \div 232333\)
Answer:
write a letter to the presiding member of your district assessment telling him or her about two of the achievement of your community over the last five years and the plans for the future
An experiment was set up as diagrammed below to measure the amount of O2 (red) and CO2 (blue) over time using live Spinach leaves and sensor probes for these gases. The results from this experiment are graphed for you. SARRON GOOD GNYGA fo a Figure 1 0.8- 06 04- 10 15 Time (min) 205 204 203 202 201 0 10 15 Time (min) (a) State which metabolic process occurred in this apparatus. (b) Explain the graphed results related to that process.
Answers
The metabolic process that occurred in the apparatus of the experiment given in the question is photosynthesis. The graphed results of the experiment are related to the process of photosynthesis.
Photosynthesis is the process in which the green plants use the energy of sunlight to convert carbon dioxide and water into glucose and oxygen. In this process, chlorophyll pigment, present in the chloroplasts of the plant cells, captures the energy of sunlight. This captured light energy is used to convert water and carbon dioxide into oxygen and glucose.
In the experiment mentioned above, the metabolic process that occurred in the apparatus is photosynthesis. It is because spinach leaves were used in the experiment to observe the amount of oxygen (O2) and carbon dioxide (CO2) present in the leaves.
The graphed results of this experiment show that the amount of oxygen in the leaves increased over time while the amount of carbon dioxide decreased. This is because the leaves absorbed carbon dioxide from the air and converted it into glucose and oxygen through the process of photosynthesis. As a result, the amount of oxygen increased over time, and the amount of carbon dioxide decreased. Hence, it can be concluded that the graphed results of the experiment are related to the process of photosynthesis.
More on photosynthesis: https://brainly.com/question/29764662
#SPJ11
Which of the following is different in two isotopes of the same element?
the number of atoms
the number of electrons
the number of neutrons
the number of protons
Answers
Answer:
Different number of neutrons
Explanation:
Isotopes are different versions of an element, in which the only difference is that they have different numbers of neutrons in the nucleus, and therefore slightly different atomic masses.
what protic solvent for sn1 master organic chemistry?
Answers
Some common protic solvents used in SN1 reactions include water, alcohols, and carboxylic acids.
In organic chemistry, SN1 reactions are a type of substitution reaction that occurs in a protic solvent.
A protic solvent is a solvent that has a hydrogen atom bonded to an electronegative atom, such as oxygen or nitrogen. This allows the solvent to form hydrogen bonds with the reactants and stabilize the transition state of the reaction.
These solvents are able to stabilize the carbocation intermediate that is formed during the reaction, which helps to increase the reaction rate.
It is important to note that the choice of solvent can have a significant impact on the rate and outcome of the reaction. For example, a polar protic solvent like water will favor the SN1 reaction, while a polar aprotic solvent like acetone will favor the SN2 reaction.
To know more about protic solvents here:
https://brainly.com/question/29871726#
#SPJ11
What is the molality of a solution that contains 96 g of Calcium chloride and 450 ml of water?
Answers
The solution has a molality of 1.921 mol/kg.
What is molality?The number of moles of solute dissolved in one kilogram of solvent is known as the molality, which serves as a measurement of a solution's concentration.
How do you determine it?We must first count the moles of calcium chloride (CaCl₂) present in the solution. CaCl₂ has a molar mass of 111 g/mol.
Number of moles of CaCl₂ = 96 g / 111 g/mol = 0.8649 mol.
The kilogram mass of the solvent (water) must then be calculated. The density of water, 1 g/mL, allows us to translate the amount of water provided in milliliters (mL) to kilograms (kg).
Mass of water = 450 mL x 1 g/mL = 450 g = 0.45 kg
We can now determine the solution's molality:
Molality = moles of solute / mass of solvent in kg
Molality = 0.8649 mol / 0.45 kg = 1.921 mol/kg
As a result, the solution has a molality of 1.921 mol/kg.
To know more about molality, visit:
brainly.com/question/26921570
#SPJ1
What is the molecular formula for a compound that is 33.38% sulfur and 66.62% oxygen and has a molar mass of 192.14g?
What is the molecular formula for a compound that is 26.37 % carbon, 5.541% hydrogen, 52.70 % oxygen and 15.38% nitrogen and has a molar mass of 182.16g?
Answers
Explanation:
HOPE IT WILL HELP YOU TO SOLVE YOUR PROBLEM


The molecular formula for a compound that is 33.38% sulfur and 66.62% oxygen and has a molar mass of 192.14 g is SO₄, and the molecular formula for a compound that is 26.37% carbon, 5.541% hydrogen, 52.70% oxygen, and 15.38% nitrogen and has a molar mass of 182.16 g is C₉H₈NO₃.
What is the significance of the molecular formula?The molecular formula of a compound is a way to represent the number and types of atoms that make up a molecule and is used to identify and distinguish between different compounds, which is important in chemistry and biochemistry as it can help predict the compound's physical and chemical properties.
Hence, the molecular formula for a compound that is 33.38% sulfur and 66.62% oxygen and has a molar mass of 192.14 g is SO₄, and the molecular formula for a compound that is 26.37% carbon, 5.541% hydrogen, 52.70% oxygen, and 15.38% nitrogen and has a molar mass of 182.16 g is C₉H₈NO₃.
Learn more about the molecular formula here.
https://brainly.com/question/28647690
#SPJ2
which of the following compounds have integer i values? (select all that apply.) which of the following compounds have integer i values? (select all that apply.) kcl ch3cooh na2co3 hclo4
Answers
The following compounds have integer i values:KClNa2CO3
The following compounds have integer i values: KCl and Na2CO3.
i stands for van't Hoff factor and is the number of particles formed from one solute molecule in a solution.
An i value of 1 means that no particles are produced upon solvation or that the solute molecule exists independently in the solvent.
Conversely, an i value greater than one indicates that one molecule of solute produces more than one particle in the solvent. This includes molecules that dissociate into ions, form complexes, or exist in a solvent as aggregates.
It is worth noting that the i value is an experimentally derived value and may not necessarily be an integer.
According to the question, the following compounds have integer i values:KClNa2CO3
Learn more about molecule
brainly.com/question/32298217
#SPJ11
26 grams of 2,2 dimethyl hexane( C8H16) under goes combustion. How
much space would the CO2 gas occupy at 26 Celsius and 101.75 kPa?
Answers
Answer: The \(CO_{2}\) gas occupies a space of 5.57 L at 26 Celsius and 101.75 kPa.
Explanation:
Given: Mass = 26 g
Pressure = 101.75 kPa
Convert kPa into atm as follows.
\(1 kPa = 0.00986923 atm\\101.75 kPa = 101.75 kPa \times \frac{0.00986923 atm}{1 kPa}\\= 1 atm\)
Temperature = \(26^{o}C = (26 + 273) K = 299 K\)
Now, moles of a substance is defined as mass of substance divided by its molar mass.
As molar mass of 2,2 dimethyl hexane is 114.23 g/mol. So, its number of moles are calculated as follows.
\(No. of moles = \frac{mass}{molar mass}\\= \frac{26}{114.23 g/mol}\\= 0.227 mol\)
Formula used to calculate the volume is as follows.
PV = nRT
where,
P = pressure
V = volume
n = no. of moles
R = gas constant = 0.0821 L atm/mol K
T = temperature
Substitute the values into above formula as follows.
\(PV = nRT\\1 atm \times V = 0.227 mol \times 0.0821 L atm/mol K \times 299 K\\V = 5.57 L\)
Thus, we can conclude that the \(CO_{2}\) gas occupies a space of 5.57 L at 26 Celsius and 101.75 kPa.
Which of the following best defines a scientific theory?
A) An 'if, then' statement that can be tested by science.
B) A model used to explain how or why a phenomenon occurs.
C) A unifying concept; often a mathematical description of the way in which a natural phenomenon occurs.
D) A piece of knowledge about the outside world received through the senses or instrumentation.
E) Something that is known to be consistent with reality; that which has not been falsified.
Answers
Answer: B) A model used to explain how or why a phenomenon occurs.
Explanation: Scientific theory explain through models will educate students more. they can learn in both audio visual ways and keep that situation in brain always. a model or a blue print is a better way of educating on scientific theory as the aim. material, observation and conclusion can be derived by actually viewing the phenomenon.
Explain what is chemistry in 4 or more sentences
Answers
Answer:
the branch of science that deals with the identification of the substances of which matter is composed. the investigation of their properties and the ways in which they interact, combine, and change; and the use of these processes to form new substances.the science that deals with the properties, composition, and structure of substances (defined as elements and compounds), the transformations they undergo, and the energy that is released or absorbed during these processes.the science that deals with the properties, composition, and structure of substances (defined as elements and compounds), the transformations they undergo, and the energy that is released or absorbed during these processes.
Explanation:
When electricity is produced from coal the chemical energy in coal is changed to ___ energy
Answers
Answer: electrical energy
Explanation:
According to law of conservation of energy, energy remains conserved. It only transforms from one form to another.
Chemical energy is the energy stored in the bonds of molecules.
Electrical energy is the energy possessed by a body by virtue of movement of electrons.
Thus when When electricity is produced from coal the chemical energy in coal is changed to electrical energy
49)Solid sodium metal reacts violently with water, producing hydrogen gas (H2) and sodium hydroxide. How many moles of hydrogen gas are formed when 15.3 g of sodium are added to water?
Answers
Answer:
Number of moles of hydrogen gas produced when 15.3 g of sodium reacts with water = 0.333 moles of hydrogen gas
Explanation:
The reaction between sodium metal and water is given by the chemical equation below:
2Na(s) + 2H₂O(l) ------> 2NaOH(aq) + H₂(g)
From the equation of reaction above, 2 moles of sodium reacts with 1 mole of water to produce 2 moles of sodium hydroxide and 1 mole of hydrogen gas.
mole ratio of sodium and hydrogen gas is 2:1
molar mass of sodium =23 g/mol:
number of moles of sodium present in 15.3 g = mass/molar mass
number of moles of sodium present in 15.3 g = 15.3 g/ 23 g/mol = 0.665 moles
number of moles of hydrogen gas produced = 0.665 * 1/2 = 0.333 moles
Therefore, number of moles of hydrogen gas produced when 15.3 g of sodium reacts with water is 0.333 moles
How does the temperature change when a layer of glass is added?
Answers
Answer:
thermal shock
Explanation:
the temperatures inside the glass jar should have continued to increase over time. Internal stresses due to uneven heating. This is also known as “thermal shock”.
In general, the thicker the glass, the more prone it will be to breaking due to the immediate differences in temperature across the thickness of glass.
Borosilicate glass is more tolerant of this, as it has a higher elasticity than standard silicon glass.
You may also note that laboratory test tubes and flasks are made with thinner walls, and of borosilicate glass, when designated for heating.
Question 1
1. Na2O + H20 --->
NaOH
A. Single Replacement
B. Double Replacement
c. Decomposition
D. Synthesis
E. Combustion
Answers
Answer: D. Synthesis
Explanation:
Sodium Oxide + Water = Sodium Hydroxide
A sample of neon gas has a volume of 7.2 mL at a pressure of 1.5atm. What is the pressure exerted by the gas if the volume is increased to 28.8 mL at constant tempature
Answers
The pressure exerted by the neon gas, when the volume is increased from 7.2 mL to 28.8 mL at constant temperature, can be calculated using Boyle's Law. The pressure exerted by the neon gas, when the volume is increased to 28.8 mL at constant temperature, is 0.375 atm.
Boyle's Law states that at constant temperature, the product of the pressure and volume of a gas remains constant. Mathematically, it can be expressed as P₁V₁ = P₂V₂. This law allows us to calculate the change in pressure when the volume changes.
In this case, the initial volume (V₁) is given as 7.2 mL, and the initial pressure (P₁) is 1.5 atm. The final volume (V₂) is 28.8 mL. By substituting these values into Boyle's Law equation, we can solve for the final pressure (P₂).
When we perform the calculations, we find that the pressure exerted by the neon gas, when the volume is increased to 28.8 mL, is 0.375 atm. As the volume increases, the pressure decreases due to the inverse relationship between pressure and volume.
Using Boyle's Law: P₁V₁ = P₂V₂
Given:
Initial volume (V₁) = 7.2 mL
Initial pressure (P₁) = 1.5 atm
Final volume (V₂) = 28.8 mL
To find the final pressure (P₂):
P₂ = (P₁ * V₁) / V₂
= (1.5 atm * 7.2 mL) / 28.8 mL
= 0.375 atm
Therefore, the pressure exerted by the neon gas, when the volume is increased to 28.8 mL at constant temperature, is 0.375 atm.
for such more questions on pressure
https://brainly.com/question/24719118
#SPJ8
PLSSS HEALP ASAP!!!! WILL REWARD
A) How many moles of CO will react with 1.75 moles of Fe2O3?
B) What was the mole ratio of CO to Fe2O3?
Answers
A)1.75×3 moles of carbon monoxide
B)2:3
A)each mole of ferric oxide requires 3 moles of carbon monoxide. Therefore 1.75 moles requires 1.75 ×3 moles of carbon monoxide
When 13.5 grams of methane (ch4) burns in 40.0 grams of oxygen, how many grams of water are formed?
Answers
22.5 grams H2O are formed when 13.5 grams of methane (ch4) burns in 40.0 grams of oxygen.
What is methane?
Methane, colourless, odourless gas that occurs abundantly in nature and as a product of certain human activities. Methane is the simplest member of the paraffin series of hydrocarbons and is among the most potent of the greenhouse gases. Its chemical formula is CH4.
Where do methane emissions come from?
According to the EPA, globally, 50 to 65% of total methane emissions come from the following human-caused activities:
Raising livestockLeaks from natural gas systemsLandfills and waste from homes and businessesLearn more about Methane
https://brainly.com/question/9383711
#SPJ4
Which of the following are soluble in water: salt, gravel, or pepper
Answers
How many representative particles are there in 10.43g of sugar (C12H22011)?
Answers
Answer:
10.43 / 342 × 6.02 × 10 ^23 = 18.34 × 10^ 21