The force applied to a machine by a users is called the
Answers
Answer:
When you use a machine, you apply force to the machine. This force is called the input force. The machine, in turn, applies force to an object. This force is called the output force.
it's called the input force
Related Questions
The sum of all the potential energy and kinetic energy in an object is equal to it's
Answers Choices: A. Thermal Energy B. Kinetic Energy C.Potential Energy D. Force PLEASE HELP DUE TODAY
Answers
Answer:
B. kinetic energy
Explanation:
The sum of kinetic energy and potential energy of an object is its total mechanical energy.
Hands moving on a battery-operated clock is an example of what kind of
energy conversion?
A. Heat energy being converted to gravitational potential energy
B. Gravitational potential energy being converted to heat energy
C. Chemical potential energy being converted to kinetic energy
D. Kinetic energy being converted to chemical potential energy
Answers
Answer:
Chemical potential energy being converted to kinetic energy
Explanation:
Which reaction occurs at the anode of a galvanic cell that has a zinc
electrode in an electrolyte with zinc ions and a copper electrode in an
electrolyte with copper ions? The reduction potential for the reduction of Cu2+
= 0.34 V. The reduction potential for the reduction of Zn2+ = -0.76 V.
A. Zn(s) → Zn2+(aq) + 2e
B. Cu(s) → Cu2+(aq) + 2e
C. Zn2+ (aq) + 2e → Zn(s)
D. Cu2+ (aq) + 2e →
Cu(s)
Answers
Answer: The reaction that occurs at anode is \(Zn(s)\rightarrow Zn^{2+}(aq.)+2e^-\)
Explanation:
Given : \(E^o_{Zn^{2+}/Zn}=-0.76V\)
\(E^o_{Cu^{2+}/Cu}=+0.34V\)
The substance having highest positive reduction potential will always get reduced and will undergo reduction reaction. Here, copper will undergo reduction reaction will get reduced.
The substance having highest negative reduction potential will always get oxidised and will undergo oxidation reaction. Here, zinc will undergo oxidation reaction will get oxidised.
Oxidation reaction occurs at anode and reduction reaction occurs at cathode.
Oxidation half reaction (anode) : \(Zn(s)\rightarrow Zn^{2+}(aq.)+2e^-\)
Reduction half reaction (cathode) : \(Cu^{2+}(aq.)+2e^-\rightarrow Cu(s)\)
by knowing that hexane has the molecular formula c6h14 and cyclohexane has the molecular formula c6h12, it becomes clear that the formation of a ring resulted in the molecule having 2 fewer hydrogen atom. with this in mind, how many rings does an alkane have if its formula is c10 h16 ?
Answers
The alkane with the molecular formula C10H16 has one ring. This ring structure reduces the number of hydrogen atoms by two compared to the corresponding straight-chain alkane.
To determine the number of rings in an alkane with the formula C10H16, we can compare it to the corresponding straight-chain alkane, which would be C10H22. Since the alkane C10H16 has 2 fewer hydrogen atoms than its straight-chain counterpart, it suggests the presence of one ring in the molecule.
The molecular formula of an alkane provides information about the number of carbon and hydrogen atoms in its structure. In the given formula C10H16, the presence of 10 carbon atoms indicates that it forms a chain of carbon atoms. However, since it has 16 hydrogen atoms, which is 2 fewer than the maximum number of hydrogen atoms possible for a straight-chain alkane with 10 carbon atoms, there must be a structural deviation. The deviation in the number of hydrogen atoms suggests the formation of one ring in the molecule. Each carbon atom in the ring contributes two hydrogen atoms, whereas in a straight-chain alkane, each carbon atom is bonded to three hydrogen atoms. By forming a ring, two of the carbon atoms in the chain lose one hydrogen atom each, resulting in a net loss of two hydrogen atoms.
Therefore, the alkane with the molecular formula C10H16 has one ring. This ring structure reduces the number of hydrogen atoms by two compared to the corresponding straight-chain alkane. It is important to note that the specific arrangement of the carbon atoms in the ring and their connectivity would determine the isomeric form of the molecule.
To learn more about straight-chain alkane click here:
brainly.com/question/22403008
#SPJ11
Write the formulas for the following ionic compounds.
potassium iodide
magnesium phosphide
silver sulphide
iron(III) bromide
magnesium hydroxide
sodium sulphate
lead (IV) nitrate
ammonium carbonate
Answers
Answer:
1) KI
2) Mg3P2
3) Ag2S
4) FeBr3
5) Mg(OH)2
6) Na2SO4
7) Pb(NO3)4
8) (NH4)2CO3
how many valence electrons does each atom in an nh3 molecule contribute to the total valence electrons? a) n contributes 3, and each h contributes 1. b) n contributes 5, and each h contributes 1. c) n contributes 5, and each h contributes 3. d) n contributes 7, and each h contributes 1
Answers
In ammonia, the nitrogen shares 3 of its valence electrons with each hydrogen atom and each of the hydrogen atom shares one valence electron. So option a is right.
Nitrogen has 5 electrons in its outer shell and hydrogen has one electron. All atoms try to complete octet electronic configuration to become stable. So covalent compound forms covalent bonds by sharing the electrons. Here one nitrogen forms covalent bonds with three hydrogen atoms by sharing one electron with each.
As there is 5 electrons in the outer shell, two of them remains as lone pair of electrons. Here since only one pair of electrons are shared between atoms, they form single bond with each other.
So nitrogen shares 3 electrons and each hydrogen contributes one.
For more information regarding covalent bonds, kindly refer
https://brainly.com/question/19382448
#SPJ4

Hey besties can y'all help me out
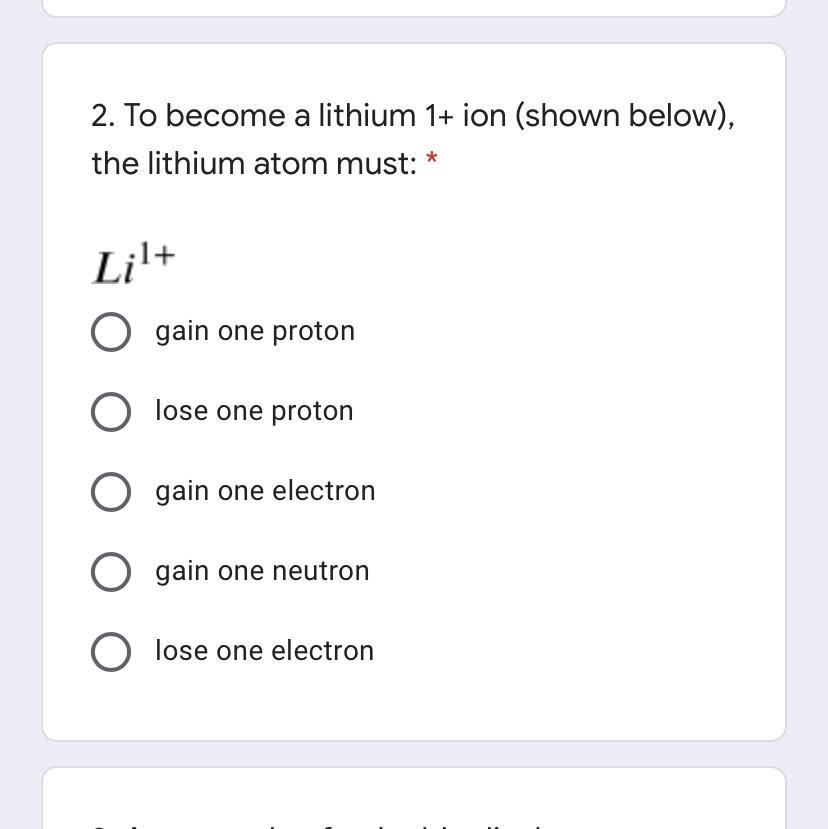
Answers
you need to lose one electron
what is upthrust? name the factor that affect the upthrust.
Answers
The upthrust keeps a ship afloat. The upthrust keeps swimmers on top of the water. Things weigh less in water.
The upward force exerted on a body by the fluid in which the body is submerged is called the upthrust or buoyant force. The property of liquid to exert an upward force on a body immersed in it is called buoyancy. Being a force upthrust is measured in newton or kgf in the system of international units. Examples Pushing an empty can into water experiences the buoyant force. Pushing a cork into water into water experiences the buoyant force. Reason for Upthrust The thrust ating on on the sides of walls of the body get neutralised because they are equal in magnitude and opposite in the directions. There is a pressure difference between the lower and upper faces of block. Since force is the product of pressure and area, The difference in pressure due to liquid is on th two faces of causes a net upward force called upthrust on the body immersed in the fluid.
Answer: upthrust an upward force exerted by a fluid that opposes the weight of an immersed object.It is the force that pushes an object up. ... the upward force that a liquid or gas exerts on a body floating in it.
When a body is placed in water, Up thrust = V x d x g
From the above formula we can say that the following are the factors that affect up thrust:
Acceleration due to gravity - (g)
Density of the liquid - (d)
Volume of the body submerged in the liquid - (V), or volume of the liquid displaced (v)
Explanation:
(0.003999 X 10^8) + (6.00111 X 10^8)
Answers
Answer:
6.005109 x 10⁸
Explanation:
Given expression:
(0.003999 x 10⁸) + (6.00111 x 10⁸)
Now, to solve for this sum,
take the coefficient and sum:
= (0.003999 + 6.00111) x 10⁸
= 6.005109 x 10⁸
In this kind of problem, just make sure the exponent parts are the same then add the coefficient together.
The list shows a number of common chemical substances. [5]
A. iron
B. carbon dioxide
C. mercury
D. hydrogen
E. sodium chloride
F. water
4 | Page
- Divide the above elements into metals and non-metals.
Answers
Answer: Not sure ;c
Explanation:
A student bought a 1.55-ounce chocolate bar and left it in a car on a hot day.
How many ounces of chocolate are in the melted bar?

A.
Exactly 1.55 ounces

B.
An unknown number of ounces

C.
At least 1.55 ounces

D.
Less than 1.55 ounces
Answers
Answer:
A. Exactly 1.55 ounces
the ionization energies (kj/mol) of hydrogen (h) , nitrogen (n) , sodium (na) , and oxygen (o) are 1,312, 1,402, 496, and 1,314, respectively. which element combination is least likely? responses
Answers
The least likely element combination would be hydrogen (H) and sodium (Na) since their ionization energies differ significantly.
To determine the least likely element combination, we need to consider the ionization energies and their relative values. The element combination that is least likely would involve elements with similar or close ionization energies.
Comparing the ionization energies:
1,312 kJ/mol (H) < 1,402 kJ/mol (N) < 1,314 kJ/mol (O) < 496 kJ/mol (Na)
Based on these values, the least likely element combination would be hydrogen (H) and sodium (Na) since their ionization energies differ significantly.
Learn more about element here
https://brainly.com/question/31950312
#SPJ11
What would happen on the Earth if the Sun’s radiation could not travel through space?
Answers
Answer:
We'd all freaking freeze to death and be standing there like the black beetles trend.
Explanation:
This is a joke, please don't take this seriously.
Order: Streptomycin 0.25g
Available: A vial of Stroptomycin powder. Directions: Reconstitute with 9mL of sterile water for a concentration of 400mg/2mL.
a. What is the order?
b. What is the available?
c. How many mL will be administered?
Answers
A- The order is to administer Streptomycin 0.25g.
b. The available is a vial of Streptomycin powder, which can be reconstituted with 9 mL of sterile water to obtain a concentration of 400 mg/2 mL.
c. The patient will be administered a volume of 1.25 mL of the reconstituted Streptomycin solution.
To calculate the volume to be administered, we first convert the ordered dosage from grams to milligrams: 0.25g = 250mg.
Next, we use the concentration of the reconstituted solution, which is 400 mg/2 mL. This means that 2 mL of the solution contains 400 mg of Streptomycin. We set up a proportion to find the volume (X mL) that contains the required 250 mg:
400 mg / 2 mL = 250 mg / X mL
Cross-multiplying and solving for X, we get:
400 mg * X mL = 2 mL * 250 mg
X = (2 * 250) / 400 = 500 / 400 = 1.25 mL
learn more about Streptomycin here:
https://brainly.com/question/28101573
#SPJ11
Whats the density of mass?
Answers
Answer:
it depends on the mass of the object but sometimes smaller objects have more density the higher the mass the the lower the density
The amount of mismanaged plastic globally is 31. 9 million tonnes per year. If the UScontributes. 022 million tones per month, what percentage of the globalmismanaged plastic comes from the US? Show your work
Answers
To calculate the percentage of global mismanaged plastic that comes from the US, we can follow these steps:
Step 1: Convert the US contribution from tons per month to tons per year.
US contribution = 0.022 million tons per month * 12 months = 0.264 million tons per year
Step 2: Calculate the percentage.
Percentage = (US contribution / Global mismanaged plastic) * 100
Percentage = (0.264 million tons per year / 31.9 million tons per year) * 100
Calculating this expression gives:
Percentage = 0.828 %
Therefore, the US contributes approximately 0.828% of the global mismanaged plastic.
To know more about mismanaged plastic click this link -
brainly.com/question/29991316
#SPJ11
suppose the sample of magnesium used in this lab was contaminated with another metal that does not react with hydrochloric acid. how would this have changed your results?
Answers
If the sample of magnesium used in a lab was contaminated with another metal that doesn't react with hydrochloric acid, then the results obtained in the experiment would be affected.
This is because the data collected during the experiment would reflect the reaction between hydrochloric acid and the contaminated sample instead of pure magnesium. As a result, the following changes in results might have been observed:
1. The mass of the contaminated sample would be higher than the mass of pure magnesium.
2. The rate of reaction between the contaminated sample and hydrochloric acid would be slower than the reaction between pure magnesium and hydrochloric acid.
3. The volume of hydrogen gas collected from the reaction would be lower than the volume of hydrogen gas collected in the reaction between pure magnesium and hydrochloric acid.
learn more about contaminated here
https://brainly.com/question/465199
#SPJ11
Which formulas represent one ionic compound and one molecular compound? a. N2 and SO2 b. Cl2 and H2S c. BaCl2 and N2O4 d. NaOH and BaSO4
Answers
One ionic compound and one molecular compound are NaOH and BaSO₄. The correct answer is d.
NaOH represents an ionic compound, sodium hydroxide, where sodium (Na) is a metal cation and hydroxide (OH) is a polyatomic anion.
BaSO₄ represents a molecular compound, barium sulfate, where barium (Ba) is a metal cation and sulfate (SO₄) is a polyatomic anion. In molecular compounds, the atoms are held together by covalent bonds.
The other options, a) N₂ and SO₂, b) Cl₂ and H₂S, and c) BaCl₂ and N₂O₄, all consist of molecular compounds only.
Therefore, the pair of compounds that includes one ionic compound (NaOH) and one molecular compound (BaSO₄) is option d) NaOH and BaSO₄.
To know more about ionic compound, refer here:
https://brainly.com/question/15605090#
#SPJ11
Destiny prefers investments that offer the highest possible returns, even if they are risky. Which of the following bonds is Destiny most likely to be interested in?
Answers
Since Destiny is mostly interested in higher return, he will hence most likely invest in bonds with very high risk rate. Hence, Destiny will most likely be interested in Junk bond.
Both Municipal and savings bond are usually backed up by the government as as such they are safer to invest in. However, they have low rate of return. Corporate bonds are usually issued by profit - oriented companies and organizations and have a higher rate of return than municipal and savings bond. Junk bonds are a form of corporate bond with very high level of risk which is compensated by a very high return rate compared to other corporate bonds. There is a higher tendency that companies that issue junk bonds will default on their debt.Therefore, Destiny will most likely be interested in a Junk bond.
Learn more:https://brainly.com/question/17405470?referrer=searchResults
Options:
- Saving bonds
- Corporate bonds
- Junk bonds
- Municipal bonds
Answer: Junk bonds
An electron moved from shell n = 2 to shell n = 1. What most likely happened during the transition? (4 points)
A fraction of a photon was added.
A photon of energy was absorbed.
A fraction of a photon was removed.
A photon of energy was released.
Answers
FILL THE BLANK. The least reactive metals are found _____ of the activity series, while the most reactive metals are found _____.
a. in the middle; at the top
b. at the top; at the bottom
c. at the top; in the middle
d. at the bottom; at the top
Answers
The least reactive metals are found at the bottom of the activity series, while the most reactive metals are found at the top. Option D.
The activity series is a ranking of the reactivity of metals, in which the least reactive metals are placed at the bottom and the most reactive metals are placed at the top. The activity series is based on the tendency of metals to lose electrons, and the reactivity of a metal is inversely proportional to its position in the series.
Therefore, the least reactive metals are found at the bottom of the activity series, where they are least likely to lose electrons, while the most reactive metals are found at the top of the series, where they are most likely to lose electrons.
Learn more about metals
https://brainly.com/question/29404080
#SPJ4
_________ is the movement of materials across a cell membrane using cellular energy.
Passive Transport
Active Transport
Answers
Answer:
active transport
Explanation:
passive transport does not involve energy
the volume of a gas at 5.0 atm is 3.5 l. what is the volume of the gas at 7.0 atm at the same temperature?
Answers
At the same temperature, the gas has a 2.5 L volume at 7.0 atm. Volume in the context of gases is the quantity of space a gas takes up.
The ideal gas law, which connects a gas's pressure, volume, and temperature, can be used to solve this issue. PV = nRT. Assuming that the temperature stays constant, we may apply the ideal gas law in the following manner: P1V1 = P2V2. With the supplied values plugged in, we obtain (5.0 atm)(3.5 L) = (7.0 atm) (V2). We can calculate V2 as follows: V2 = (5.0 atm)(3.5 L) / (7.0 atm) = 2.5 L. As a result, the gas has a 2.5 L volume at the same temperature at 7.0 atm. In many industrial, scientific, and medical applications, accurate gas volume measurement and management are crucial.
Learn more about Volume here:
https://brainly.com/question/28368781
#SPJ4
CAN SOMEONE HELP ME!!!!!!!!!!!!!

Answers
Answer:
5 g/cm^3
Explanation:
Density = mass / volume
What is chemistry the study of?
Answers
1. You are in the lab. You perform the following chemical reaction by mixing a piece of magne
applying heat and oxygen. Balance the following chemical equation that represents this rea
2
0₂ →
Mg+
MgO
Mg=12
Mg-x2
0 +2
2. What is the mole to mole ratio of Mg to MAO in the chemical equation above?
mol Mg:
mol MgO
3. Calculate the molar mass of the following (1 pt ea.):

Answers
2Mg + O2 → 2MgO
2. The mole to mole ratio of Mg to MgO is 2:2 or 1:1. This means that for every 1 mole of Mg that reacts, 1 mole of MgO is produced.
mol Mg: mol MgO = 1:1
3. Molar mass of the following:
- NaCl (sodium chloride) = 22.99 g/mol + 35.45 g/mol = 58.44 g/mol
- H2SO4 (sulfuric acid) = 2(1.01 g/mol) + 32.06 g/mol + 4(16.00 g/mol) = 98.08 g/mol
- Ca(NO3)2 (calcium nitrate) = 1(40.08 g/mol) + 2(14.01 g/mol + 3(16.00 g/mol)) = 164.09 g/mol
- C6H12O6 (glucose) = 6(12.01 g/mol) + 12(1.01 g/mol) + 6(16.00 g/mol) = 180.18 g/mol
What mass of hydrogen is produced from the reaction of 50.0 g of Mg and 75.0 g of HCl? How much of the excess reagent is left over?
Mg + 2HCl → MgCl2 + H2
Answers
Someone PLEASE help me I don’t remember this.

Answers
Answer: genotype: Ee phenotype: two small eyes.
genotype: RR' phenotype: pink eyes
genotype: GB phenotype: green and blue splotches
genotype: cc phenotype: straight
genotype: Tt phenotype: has tail
genotype: Ss phenotype: sharp teeth
genotype: FF' phenotype: three toes
genotype: ww phenotype: white
genotype: YY phenotype: pointy
genotype: nn phenotype: two ears
genotype: Ll phenotype: long
Explanation: hope this helps (the uppercase letters are dominant genes. the lowercase letter are recessive genes. for a recessive gene to show up in a phenotype you need 2 lower case letters such as cc or ss. for a dominant gene to show up in phenotype you need either 1 or 2 uppercase letters such as Cc or SS. Codominant genes present both colors in the phenotypes i.e. a brown and white cow. incomplete dominance is when neither gene is dominant so a mix of the 2 are present in the phenotype i.e. a pink rose. A regulatory gene controls the expression of a gene
Find the yield of Cu(OH)2 using CuCl₂
Answers
The yield of Cu(OH)2 is equal to the amount of CuCl2 used.
What is yield?Yield is a quantitative measure of the amount of product obtained from a reaction. It is usually expressed as a percentage of the theoretical yield, which is the maximum amount of product that could be obtained from the given amounts of reaction components. Yield is an important concept in chemistry because it provides a means for evaluating the efficiency of the reaction, the quality of the conditions and the effectiveness of the purification process. Yield can be determined by measuring the amount of product obtained following a reaction, then dividing that amount by the theoretical yield. This calculation provides an estimate of how much of the desired product was obtained from the reaction.
The yield of Cu(OH)2 using CuCl2 is determined by the mole ratio of the two reactants. The balanced equation for the reaction is:
CuCl2 + 2NaOH → Cu(OH)2 + 2NaCl
The mole ratio of CuCl2 to Cu(OH)2 is 1:1, so for every mole of CuCl2 used, one mole of Cu(OH)2 is produced. Therefore, the yield of Cu(OH)2 is equal to the amount of CuCl2 used.
To learn more about yield
https://brainly.com/question/14531883
#SPJ1
the study of the relationships between the quantities of reactants and products involved in chemical reactions is called _________.